Introduction: Why Nitrosamine Testing in Lapatinib Matters
Nitrosamine testing in lapatinib has become crucial for ensuring the safety of cancer treatments. Lapatinib is a medication used mainly for treating HER2-positive breast cancer. Due to its complex chemical structure and multiple steps involved in its production, it has a higher chance of forming harmful nitrosamine impurities. These impurities are closely monitored by health authorities like the FDA and EMA. At ResolveMass Laboratories, we use advanced testing techniques to detect nitrosamines early, helping drug makers stay compliant and protect patient health.
Understanding How Lapatinib Can Form Nitrosamines
Lapatinib has a detailed chemical name and structure that includes several types of amines, such as secondary and tertiary amines. These amines can react under certain conditions—like in acidic or oxidative environments—and turn into dangerous substances called NDSRIs (Nitrosamine Drug Substance-Related Impurities). Because of this, it’s important to examine the structure of lapatinib carefully throughout the drug development process to avoid the formation of these impurities.
Synthesis of Lapatinib: An Overview
Lapatinib is synthesized through a multi-step chemical process involving key reactions such as nucleophilic substitution, amide bond formation, and heterocyclic ring construction. The process includes the coupling of substituted aniline and quinazoline derivatives. Careful control of reaction conditions is essential to minimize the formation of impurities, including potential nitrosamines.
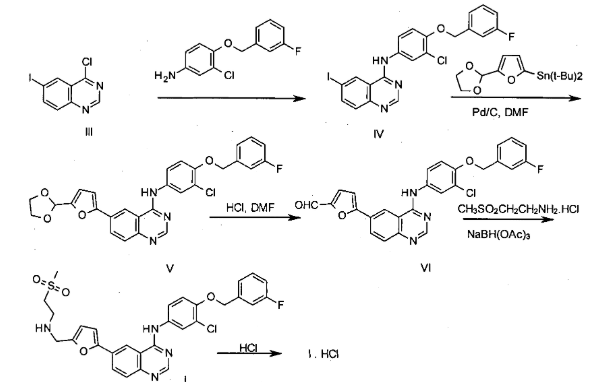
Muddasani, P. R., Talasila, S. R., Satti, V. R., Nekkanti, S. C., & Nannapaneni, V. C. (2014). Process for the preparation of lapatinib (WO2014170910A1). World Intellectual Property Organization. https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2014170910
Nitrosamine Formation Risks in Lapatinib API
1. Quinazoline- and Aniline-Derived Impurities
The synthesis pathway of Lapatinib originates from a quinazoline scaffold. Incomplete halogenation during early steps may lead to impurities such as deschloro or mono-chlorinated variants. One critical intermediate, 3-chloro-4-(3-fluorobenzyloxy)aniline, must be fully reacted due to its known genotoxicity. Additionally, structural deviations like desfluorinated or dealkylated derivatives can emerge during synthesis.
2. Impurities Resulting from Biaryl Coupling Reactions
In the biaryl coupling stage involving a furanyl moiety, suboptimal reaction conditions may result in the formation of side-products, including a formyl impurity (also known as Lapatinib Impurity 5; CAS No. 231278-84-5). The use of palladium-tin catalysts can introduce trace metal residues, while diazonium-based synthetic routes may yield by-products such as azo compounds, phenols, or nitroso analogs due to the instability of intermediates.
3. Amine-Related Impurities from Reductive Amination
During the attachment of the sulfonylethyl group through reductive amination, various by-products may arise. Incomplete reduction can result in imine intermediates, whereas over-reduction may yield corresponding alcohols. A noteworthy impurity in this stage is the des-mesylethyl analog (CAS No. 697299-82-4), along with N-hydroxy derivatives like CAS 1360431-86-2, which may form due to oxidative conditions.
4. Impurities in Final API and Salt Formation
The active pharmaceutical ingredient (API), Lapatinib, is typically isolated as a ditosylate salt. This stage may present impurities such as residual p-toluenesulfonic acid, solvent traces (e.g., DMF or acetonitrile), and undesired polymorphic forms. While these may not compromise therapeutic efficacy directly, they can significantly affect the purity profile and long-term stability of the drug substance.
5. Nitrosamine Impurities from Secondary Amine Functionality
Lapatinib contains a secondary amine moiety, making it susceptible to nitrosation reactions under specific conditions. This can lead to the formation of nitrosamine-related impurities, including N-nitroso derivatives such as impurity 1, 2, and 3, with molecular weights ranging between 610 and 639 Da. Due to their potential carcinogenicity, stringent monitoring and control strategies must be implemented throughout production.
Nitrosamine Risks During Lapatinib Manufacturing
Lapatinib ditosylate, during tablet formulation, presents a risk of nitrosamine impurity formation due to its secondary sulfonylethylamine group, which can undergo nitrosation. This risk is elevated during manufacturing steps such as granulation, drying, and compression—especially when trace nitrites from excipients like magnesium stearate or talc are present, and when the process is exposed to heat and humidity.
The most prominent impurity formed is N-Nitroso Lapatinib Impurity 1 (C₂₉H₂₅ClFN₅O₅S; MW ≈ 610), produced via direct nitrosation of the secondary amine. Additional impurities, including N-Nitroso Lapatinib Impurity 2 (C₂₉H₂₄ClFN₆O₆S; MW ≈ 639) and Impurity 3, have been observed under stress and storage conditions.
Moisture introduced during the coating stage or due to insufficient packaging can further accelerate nitrosamine formation. To mitigate these risks, manufacturers should:
- Use low-nitrite excipients
- Add antioxidants or pH modifiers to reduce nitrosation potential
- Select protective packaging that limits moisture and oxygen exposure
- Monitor impurities using validated LC-MS/MS methods
These measures are essential to ensure product safety and regulatory compliance.and complying with global nitrosamine regulatory limits.
Global Regulations on Nitrosamine Testing in Lapatinib
Regulatory Agencies and Known Nitrosamines
Health authorities around the world have strict rules when it comes to nitrosamines. For drugs like lapatinib that contain many amine groups, this is especially important. Below are some known nitrosamines linked to lapatinib:
| NDSRI Name | Source in Drug Structure | Regulatory Focus |
|---|---|---|
| N-nitroso-lapatinib | From benzimidazole structure | EMA under review |
| Nitroso-fluoroaniline | Breakdown of fluoroaniline part | Health Canada monitoring |
| Nitroso-N-methylquinazoline | Intermediate in synthesis | Restricted in EU |
Regular updates from agencies like the FDA and EMA help companies stay informed. Aligning with these guidelines is critical for market approval and patient safety.
Refer to our exhaustive guide on NDSRIs in Nitrosamine Testing.
Advanced Nitrosamine Testing in Lapatinib at ResolveMass Laboratories
Our Analytical Services
ResolveMass Laboratories provides reliable nitrosamine testing in lapatinib using top-tier equipment and methods. We use tools like LC-HRMS and GC-MS/MS, which can detect nitrosamines at very low levels. We also run forced degradation studies and use in-silico modeling to predict impurity risks. Whether it’s for early-stage drug development or a commercial batch release, our team ensures your product meets all nitrosamine-related standards.
Related APIs We Analyze
Besides lapatinib, we test many other drugs for nitrosamine risks. Some of these include:
- Nitrosamine Testing in Vonoprazan
- Nitrosamine Testing in Apixaban
- Nitrosamine Testing in Sitagliptin and Related APIs
- Nitrosamine Testing in Betahistine
- Nitrosamine Testing in Amitriptyline
And read our guide on:
Acceptable Intake of Nitrosamines: What You Need to Know
For each drug, we study its chemical pathway, possible impurities, and how to manage them. Our knowledge base contains valuable insights and is regularly updated.
Summary: The Importance of Nitrosamine Testing in Lapatinib
To keep cancer treatments safe and compliant, proper nitrosamine testing in lapatinib is essential. Key steps include:
- Identifying where nitrosamines can form in the chemical structure
- Preventing contact with nitrites at every stage of production
- Testing the drug and its materials for heat, moisture, and light sensitivity
- Using accurate and advanced tools to find even tiny amounts of nitrosamines
- Following all global regulations and keeping documentation ready for inspections
ResolveMass Laboratories is your trusted partner in this process. We help you stay compliant, reduce risk, and keep patients safe.
Contact ResolveMass for Expert Support
Need precision, expertise, and regulatory trust?
📩 Contact Us
📞 Get in Touch
🧪 Schedule Nitrosamine Testing
Frequently Asked Questions (FAQs)
Lapatinib contains chemical groups called amines, which can react with other substances like nitrites under certain conditions. These reactions can lead to the formation of harmful nitrosamines if not properly controlled.
Nitrosamines in lapatinib can be detected using advanced instruments like LC-MS/MS or GC-MS. These tools help identify and measure even very small amounts of nitrosamines to ensure the product is safe.
Yes, solvents like DMF or DMA can lead to the formation of dimethylamine, a substance that can react with nitrites to produce NDMA—a harmful nitrosamine. Proper solvent control is important to prevent this.
Risk can be reduced by using high-purity ingredients, avoiding nitrites in the process, and separating reactive compounds. Regular testing and careful process design also play a key role in prevention.
ResolveMass offers complete testing services, risk assessments, and regulatory support. From raw material analysis to final product release, our labs ensure compliance with nitrosamine safety standards.
References
- Muddasani, P. R., Talasila, S. R., Satti, V. R., Nekkanti, S. C., & Nannapaneni, V. C. (2014). Process for the preparation of lapatinib (WO2014170910A1). World Intellectual Property Organization. https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2014170910
- Control of Nitrosamine Impurities in Human Drugs