Nitrosamine Testing in Metformin has become a crucial part of pharmaceutical safety. This is due to the discovery of harmful substances like NDMA (N-nitrosodimethylamine) in several metformin-based medications. Regulatory bodies such as the FDA, EMA, and Health Canada have made it mandatory for companies to assess and control nitrosamine risks. At ResolveMass Laboratories Inc., we offer complete nitrosamine testing solutions using advanced analytical tools. Identifying and controlling these impurities early is key to meeting regulations and protecting patient health.
Understanding the Synthesis of Metformin
Metformin hydrochloride is created using a guanidine-based method. The main ingredients are dimethylamine hydrochloride (DMA·HCl) and cyanoguanidine. These chemicals react in solvents like water, ethanol, or methanol to form metformin. However, dimethylamine, one of the main ingredients, can lead to NDMA if nitrosating conditions are present. That’s why it’s important to monitor and control each step of the process carefully. Changing solvents and optimizing the chemical reaction can help reduce the chance of nitrosamine formation.
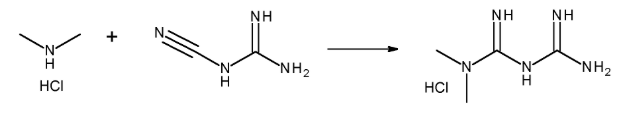
Merck Patent GmbH. (2019). Process for the preparation of metformin (WO2019154769A1). World Intellectual Property Organization. https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2019154769
Nitrosamine Testing in Metformin API Synthesis
During the API (active pharmaceutical ingredient) stage, several factors can increase nitrosamine levels. Proper Nitrosamine Testing in Metformin APIs helps in catching these risks early.
Dimethylamine Hydrochloride (DMA·HCl)
This chemical is a secondary amine and is very sensitive to forming NDMA, especially when nitrite or oxidants are present. To avoid contamination, it’s important to use pure reagents and conduct frequent testing.
Presence of Nitrites or Nitrosating Agents
Even small traces of sodium nitrite can react with DMA·HCl and form NDMA, especially under acidic conditions. To prevent this, all materials should be screened and processes controlled strictly.
Solvents That Contribute to NDMA
Solvents like DMF, DMAc, and NMP can break down into dimethylamine under heat. These leftover compounds can lead to NDMA. Choosing safer solvents or adjusting the process to prevent breakdown is recommended.
Nitrosamine Testing in Metformin Finished Products
Testing doesn’t stop at the API level. The finished tablets can also develop nitrosamines over time. This makes Nitrosamine Testing in Metformin tablets just as important.
Secondary Amines in Metformin Structure
Metformin’s chemical structure includes dimethylamino groups, which can easily form NDMA under the wrong conditions—especially during long storage or high heat.
Excipient Contamination
Some commonly used fillers like microcrystalline cellulose or lactose may have nitrites. These can react with metformin and cause NDMA buildup, especially in humid or acidic conditions.
Degradation During Manufacturing
Heat and moisture during production can break down metformin and increase dimethylamine levels. When nitrites are also present, the chance of forming NDMA rises sharply.
Impact of pH and Moisture
Acidic conditions and exposure to moisture—common in extended-release tablets—can increase the risk. Good packaging and the use of moisture absorbers (desiccants) can help.
Known Nitrosamines Found in Metformin
| Nitrosamine Impurity | Formation Source in Metformin | Detected or Plausible? |
|---|---|---|
| NDMA | From DMA·HCl + nitrite (during synthesis or from excipients) | ✅ Confirmed in multiple recalls (tablets, ER forms) |
| NMEA | Possible via ethylamine impurity and nitrosation | ⚠️ Plausible but rarely reported |
| NNG | Degradation of guanidine functional group | ⚠️ Hypothetical; not detected yet |
| NDIPA | Potential from external diisopropylamine contamination | ⚠️ Under watchlist; low relevance unless process uses DIPEA |
Regular testing using sensitive tools like LC-MS/MS ensures any nitrosamines are caught early, keeping the product safe.
Global Guidelines on Nitrosamine Testing in Metformin
The FDA began recalling metformin in 2020 when NDMA levels were too high. The EMA followed with a rule that all metformin makers must do full nitrosamine risk checks. Health Canada also asked for NDMA testing and recommended advanced LC-MS/MS methods that detect even small traces (below 96 ng/day). These global rules show how seriously nitrosamines are taken and why routine testing is essential.
🔗 For advanced guidance on regulatory thresholds and AI limits, visit:
➡️ Acceptable Intake of Nitrosamines
➡️ Risk Evaluation Support
Summary – Why Nitrosamine Testing in Metformin Matters
NDMA is the most concerning impurity found in metformin, mainly because of ingredients like DMA·HCl and possible nitrite exposure. Heat, moisture, and poor storage can all add to the problem. Regulators have made it clear that ignoring nitrosamines is not an option. At ResolveMass Laboratories Inc., we provide full Nitrosamine Testing in Metformin APIs and finished products using advanced tools that meet international standards.
💡 Explore more:
➡️ Nitrosamine Testing in Folic Acid
➡️ Nitrosamine Testing in Apixaban
➡️ NDSRI Detection Support
FAQs
NDMA forms in metformin when dimethylamine (a part of metformin’s structure or raw materials) reacts with nitrites. This reaction happens more easily in acidic or moist conditions, especially during storage. If proper controls aren’t in place, NDMA can build up over time in the final product.
Yes, the synthesis of metformin API carries a natural risk of forming nitrosamines like NDMA. This is because it uses chemicals like dimethylamine hydrochloride, which can react with nitrites. If manufacturing steps aren’t carefully controlled, this reaction may happen during production.
Solvents such as DMF (dimethylformamide), DMAc (dimethylacetamide), and NMP can break down into dimethylamine, a nitrosamine-forming substance. When these solvents are heated or hydrolyzed, they release dimethylamine, which may then react with nitrites to form NDMA.
Health agencies like the FDA and EMA have set a safety limit for NDMA at 96 nanograms per day. If a metformin product has more than this amount, it may be recalled. These strict limits help protect patients from possible long-term cancer risks linked to NDMA.
Yes, excipients like fillers and binders can contain trace levels of nitrites. These nitrites may react with dimethylamine in the API to form NDMA, especially during storage in warm or humid conditions. That’s why it’s important to test excipients for such impurities before use.
N-nitrosoguanidine (NNG) is a type of nitrosamine that could theoretically form due to guanidine groups in metformin. While it hasn’t been commonly found in metformin, it’s still considered a potential risk. Monitoring and testing help make sure it doesn’t appear in final products.
Yes, extended-release (ER) metformin tablets may have a higher risk of nitrosamine formation. This is because they are stored longer and may be more exposed to moisture and heat. These conditions can increase the chances of NDMA forming over time if not properly managed.
📞 Connect with Us — Let’s Make Metformin Safer Together
ResolveMass Laboratories Inc. offers validated Nitrosamine Testing in Metformin to ensure regulatory compliance and consumer safety. Contact our expert team for tailored CRO services and regulatory consulting:
🔗 Contact Us – Page 1
🔗 Contact Us – Page 2
🔗 Reach Out for Custom Testing
🔗 Additional Resources for Your Reference
- Nitrosamine Analysis Services
- Nitrosamine Risk Assessment Guide
- ResolveMass Analytical Laboratory
- Nitrosamine Testing in Vonoprazan
- Nitrosamine Testing in Acyclovir
References
- Merck Patent GmbH. (2019). Process for the preparation of metformin (WO2019154769A1). World Intellectual Property Organization. https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2019154769
- Control of Nitrosamine Impurities in Human Drugs
- Information about Nitrosamine Impurities in Medications


