Introduction: Why Nitrosamine Testing in Nizatidine Matters
Nitrosamine testing in Nizatidine is a vital part of ensuring drug safety and meeting international quality standards. Due to past alerts about harmful substances like NDMA in similar medications, it’s important to test every batch of Nizatidine carefully. Since Nizatidine has chemical parts that can easily react and form nitrosamines, regular testing helps catch these issues early. At ResolveMass Laboratories Inc., we specialize in advanced testing methods to help pharmaceutical companies stay compliant and protect public health. Our team focuses on accuracy and early detection to support safe product development.
Understanding the Synthesis of Nizatidine
Nizatidine is created through a series of chemical steps that involve several raw materials and processes:
- Starting Material: 2-[(dimethylamino)methyl]thiazole-4-carboxaldehyde
- Key Intermediate: Made using 2-[(nitrothio)ethyl]acetamide
- Final API: Formed by adding a dimethylaminoethyl group to the thiazole ring
During these steps, various chemicals are introduced that may lead to nitrosamine formation. Compounds like dimethylamine, nitro groups, and thiazole rings are especially sensitive to reactions that can form nitrosamines. Knowing this helps in identifying where risks begin and how to manage them effectively from the start.
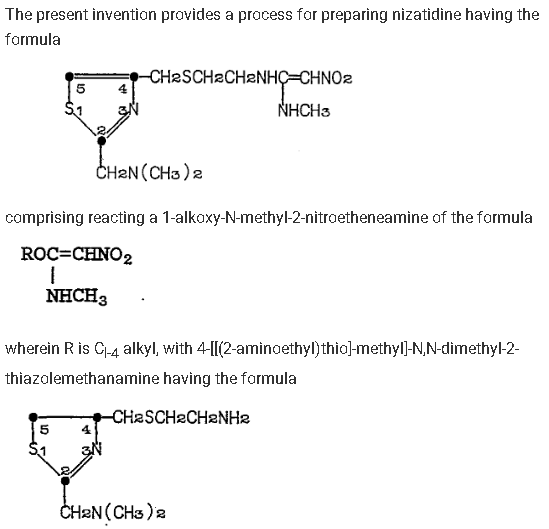
Jackson, B. G., & Slomski, B. A. (1987). Process for preparing Nizatidine (European Patent No. EP0230127A1). European Patent Office. https://worldwide.espacenet.com/patent/search/family/007785448/publication/EP0230127A1
Nitrosamine Risk Assessment in Nizatidine Manufacturing
Dimethylamine and NDMA Formation
Dimethylamine (DMA) is either added directly or formed during manufacturing, often from solvents like DMF or DMAc. When nitrites are present, even in small amounts, DMA can react and form NDMA. This is one of the main concerns in nitrosamine testing in Nizatidine, and it must be closely monitored throughout the process.
Risk from Solvent Breakdown
Solvents used in the production of Nizatidine can break down and produce amines like DMA. If nitrites are found in these solvents—possibly from impurities or poor storage—they may react to form nitrosamines. Good solvent quality and control reduce this risk significantly.
Intermediate-Related Contamination
Some intermediates, such as nitrothioethyl acetamide, can break down into reactive substances that may also turn into nitrosamines. These reactions often happen in the presence of oxidizing agents or when equipment isn’t fully cleaned between batches.
Raw Materials and Reagent Issues
Even small amounts of nitrites in raw materials or reagents can lead to nitrosamine contamination. If they come into contact with DMA or similar chemicals, NDMA may form. This highlights the need for thorough checks of all incoming materials.
Reused Solvents and Contamination Risk
When solvents like DMF or NMP are reused without proper purification, they may contain leftover substances that help form nitrosamines. This is especially risky in facilities using continuous or multi-batch production systems. Clean and fresh solvents help prevent such problems.
Rare but Important Risks
In some rare cases, substances like hydrazine or hydroxylamine may be used. These can also lead to nitrosamine formation through different chemical reactions. Though not common in Nizatidine production, it’s wise to include them in broader risk reviews.
Nitrosamine Testing in Nizatidine Finished Products
Even after the active ingredient is made, Nizatidine products can still develop nitrosamines during packaging, storage, or shelf life. That’s why nitrosamine testing in Nizatidine must continue even after the drug is finalized.
Sensitive Dimethylamino Side Chain
Nizatidine has a dimethylamino side chain, which is known to react with nitrites. These nitrites might come from packaging materials or even from air moisture. Over time, these reactions can lead to NDMA, especially if the product isn’t stored properly.
History of Recalls Due to NDMA
Back in 2019, several Nizatidine products were recalled due to NDMA contamination. These findings came from stability studies and highlighted the importance of regular nitrosamine checks in finished products. Regulatory agencies now recommend routine monitoring.
Hidden Nitrites in Excipients
Sometimes, common tablet ingredients like microcrystalline cellulose or magnesium stearate contain tiny amounts of nitrites. These can trigger nitrosamine formation when they come into contact with the active ingredient, particularly in humid conditions.
N-Nitroso-Nizatidine Formation
Nizatidine itself can form a specific nitrosamine known as N-nitroso-Nizatidine. This compound is now recognized by both FDA and EMA and is considered a critical impurity. Therefore, nitrosamine testing in Nizatidine must look for this specific impurity.
Stability Testing and Degradation
Under stress conditions like high heat and humidity, NDMA can form during shelf life. Testing under ICH Q1A(R2) conditions helps companies understand how the product breaks down and whether nitrosamines might develop over time.
Documented Nitrosamine Impurities in Nizatidine
| Impurity Name | How It Forms |
|---|---|
| NDMA (N-Nitrosodimethylamine) | From DMA or product breakdown |
| NDEA | Possibly from diethylamine in raw materials |
| N-Nitrosoethylmethylamine | A mix of methyl and ethyl amines reacting with nitrites |
| N-Nitroso-Nizatidine | A nitrosamine unique to Nizatidine’s structure |
| N-Nitrosomethyl-Nizatidine | From methyl nitrosation of the Nizatidine molecule |
These impurities can form at various stages—during production or even after packaging. That’s why complete nitrosamine testing in Nizatidine is necessary from start to finish.
Services We Offer for Nitrosamine Testing in Nizatidine
At ResolveMass Laboratories, we offer a full range of services to help you detect and manage nitrosamine risks, including:
- Testing of Metformin, Folic Acid, and Vonoprazan
- Identification of NDSRIs in raw materials and finished products
- Evaluation of regulatory intake limits for nitrosamines
- High-sensitivity analysis using GC-MS and LC-MS/MS
- Regulatory guidance and CRO support
- Custom risk assessments for your formulations
Related Reading and Risk Mitigation Resources
Explore our specialized services and scientific guides to evaluate nitrosamine risks in complex drug matrices like Nizatidine:
- ✅ Nitrosamine Testing in Metformin
- ✅ NDSRIs in Nitrosamine Testing
- ✅ Acceptable Intake Limits of Nitrosamines
- ✅ CRO Support for Nitrosamine Risk Evaluation
- ✅ Nitrosamine Risk Assessment Guide
- ✅ Nitrosamine Analysis Laboratory
- ✅ Nitrosamine Analysis Capabilities
- ✅ Nitrosamine Testing in Folic Acid
- ✅ Nitrosamine Testing in Vonoprazan
Conclusion: Ensure Safety with Nitrosamine Testing in Nizatidine
Nitrosamine testing in Nizatidine is not optional—it’s essential for patient safety and regulatory compliance. From manufacturing to packaging, the risks are real and must be managed with expert support. ResolveMass Laboratories is here to guide you every step of the way. Our advanced testing techniques and deep knowledge of nitrosamine chemistry make us the trusted partner for pharmaceutical companies worldwide.
📞 Contact us today to begin your Nitrosamine Testing in Nizatidine project and stay ahead of compliance.
📩 Contact ResolveMass
📨 Speak with Our Scientists
📬 Schedule Consultation
FAQs
Yes, Nizatidine has been found to contain NDMA (N-Nitrosodimethylamine) in some batches. NDMA is a type of nitrosamine impurity that can form during manufacturing or storage, especially under heat or moisture. Due to this, several Nizatidine products were recalled by health authorities like the FDA to ensure patient safety.
In Nizatidine synthesis, NDMA (N-Nitrosodimethylamine) is the most likely nitrosamine impurity. It can form when dimethylamine, a part of the Nizatidine structure, reacts with nitrites. Other possible impurities include N-nitroso-Nizatidine and N-nitrosoethylmethylamine, especially if contaminated solvents or nitrosating agents are present.
Yes, nitrite in excipients can react with the dimethylamino group in Nizatidine and form NDMA. Even very small amounts of nitrite in materials like microcrystalline cellulose or magnesium stearate can trigger nitrosamine formation, especially during storage or heat exposure.
The acceptable daily intake (ADI) for NDMA in medicines like Nizatidine is usually set at 96 nanograms per day by agencies such as the FDA and EMA. This limit helps reduce long-term cancer risk and is used to decide if a product is safe for patients.
No, once nitrosamines like NDMA are formed in the final Nizatidine product, they cannot be removed easily. That’s why prevention is key—by controlling raw materials, excipients, and storage conditions during manufacturing.
Yes, using recycled solvents like DMF or DMAc without proper cleaning can introduce amine residues or nitrites, which can lead to NDMA formation. Careful control and testing of recycled solvents are needed to avoid this risk.
Yes, higher temperatures can speed up the formation of nitrosamines in Nizatidine, especially during storage. This is why stability studies at elevated temperatures are required—to check if NDMA forms over time in the product.
References
- Jackson, B. G., & Slomski, B. A. (1987). Process for preparing Nizatidine (European Patent No. EP0230127A1). European Patent Office. https://worldwide.espacenet.com/patent/search/family/007785448/publication/EP0230127A1
- Control of Nitrosamine Impurities in Human Drugs
- Information about Nitrosamine Impurities in Medications


