Introduction: Why Nitrosamine Testing in Palbociclib Is Critical
Nitrosamine testing in Palbociclib has become an essential step in ensuring drug safety and regulatory compliance. Palbociclib, a widely used oral medication for treating advanced breast cancer, is at risk of forming nitrosamine impurities during its production and storage. These impurities, especially Nitrosamine Drug Substance Related Impurities (NDSRIs), are known for their potential cancer-causing properties.
To manage this risk, it’s important to understand how these impurities form during Palbociclib synthesis and formulation. This article explores the key areas where nitrosamines can emerge and explains how ResolveMass Laboratories helps detect and control them effectively.
Understanding Palbociclib Synthesis and Nitrosamine Formation
Palbociclib has a complex chemical structure built around a pyridopyrimidinone ring system. Its production involves steps like nitration, hydrogenation, and ring closure, often using solvents such as DMF and NMP. These solvents and the reagents used, including amine-based compounds, can increase the risk of nitrosamine formation if nitrosating agents are present.
Some chemical reactions require high temperatures, strong acids, or oxidizing conditions. These factors can introduce unwanted side reactions or leave behind impurities. Careful control over temperature, pH, and chemical purity is necessary to limit nitrosamine contamination during synthesis.
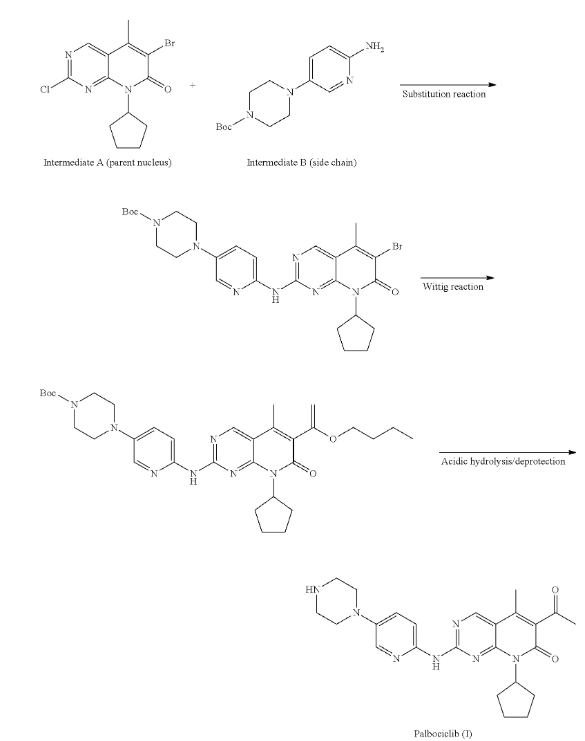
Xu, X. (2017). Method for preparing palbociclib (U.S. Patent Application No. US20170247380A1). United States Patent and Trademark Office. https://patents.google.com/patent/US20170247380A1
Nitrosamine risk assessment in Palbociclib API synthesis
The active pharmaceutical ingredient (API) stage is one of the main points where nitrosamine impurities can develop. Here are some risk areas during Palbociclib API synthesis:
- Use of DMF and NMP: These solvents can form NDMA (N-nitrosodimethylamine) when exposed to nitrosating agents.
- Amine-containing intermediates: Reagents like morpholine or piperidine may lead to nitrosamines such as N-nitrosomorpholine (NNM) or N-nitrosopiperidine (NPIP).
- Presence of nitrite compounds: Sodium nitrite and similar agents can react with amines, forming harmful compounds like NDEA or NEMA.
- Oxidizing environments: Peroxides or oxygen exposure can change precursors into nitrosamine-forming substances.
- Recycled solvents: If not purified well, recycled solvents may still contain nitrites or amines, increasing the nitrosation risk.
By validating each process step and regularly testing raw materials and solvents, manufacturers can significantly reduce these risks.
Nitrosamine Testing in Palbociclib Finished Product
Even after the API is produced, nitrosamine testing in Palbociclib continues to be important during formulation and packaging. Here’s why:
- API degradation: Under certain conditions (heat, light, moisture), the Palbociclib molecule may break down into nitrosatable fragments.
- Excipients with nitrites: Some common excipients might carry trace amounts of nitrites, which can react with residual amines.
- Storage and packaging: High humidity and temperatures can promote nitrosamine formation if materials are not properly stored.
- Cross-contamination risks: Using shared production lines for different drugs can lead to the accidental introduction of nitrosating substances.
To prevent these issues, manufacturers should implement quality control checks, select low-nitrite excipients, and use protective packaging materials.
Known Nitrosamines Found in Palbociclib Products
Various nitrosamines have been identified as potential contaminants in Palbociclib. These include:
| Nitrosamine Impurity | Possible Source |
|---|---|
| NDMA (N-Nitrosodimethylamine) | DMF/NMP solvent reactions |
| NDEA (N-Nitrosodiethylamine) | From ethanolamine or tertiary amines |
| NNM (N-Nitrosomorpholine) | Formed if morpholine is used in synthesis |
| NPIP (N-Nitrosopiperidine) | From piperidine-containing intermediates |
| NEMA (N-Nitrosoethylmethylamine) | Reaction of ethylmethylamine with nitrites |
| NDIPA (N-Nitrosodiisopropylamine) | From Palbociclib degradation in hot/humid conditions |
Each impurity has a regulatory Acceptable Intake (AI) limit, and regular updates from the EMA, USFDA, and Health Canada help guide the testing requirements.
How ResolveMass Conducts Nitrosamine Testing in Palbociclib
ResolveMass Laboratories uses advanced tools to detect even the tiniest traces of nitrosamines in both Palbociclib API and its finished product. Techniques include:
- GC-MS/MS (Gas Chromatography–Mass Spectrometry)
- LC-HRMS (Liquid Chromatography–High Resolution MS)
- QTOF-MS (Quadrupole Time-of-Flight MS)
These methods can identify nitrosamines at levels as low as 10–30 parts per billion (ppb). All testing follows international guidelines to ensure safety and compliance. Explore our nitrosamine analysis laboratory to know more.
Related Services from ResolveMass Laboratories
Besides nitrosamine testing in Palbociclib, ResolveMass offers testing for other high-risk drugs such as:
- Nitrosamine Testing in Orphenadrine
- Nitrosamine Testing in Nizatidine
- Nitrosamine Testing in Metformin
- Acceptable Intake for Nitrosamines
- Risk Evaluation CRO Services
- Guide to Nitrosamine Risk Assessment
- Nitrosamine Testing in Quetiapine
- Nitrosamine Testing in Varenicline
They also provide Acceptable Intake evaluations, custom testing protocols, and consulting on regulatory strategy to keep pharmaceutical companies audit-ready.
Conclusion: Ensure Safe Use with Nitrosamine Testing in Palbociclib
Nitrosamine testing in Palbociclib is essential for ensuring the safety of patients and complying with international pharmaceutical standards. With several potential pathways for nitrosamine formation—during synthesis, formulation, or storage—proactive testing is the best way to avoid contamination.
ResolveMass Laboratories offers expert testing services, regulatory guidance, and risk assessment tools to help pharmaceutical manufacturers meet these critical quality requirements.
Contact Us
Have questions about nitrosamine testing in Palbociclib?
Frequently Asked Questions on Nitrosamine Testing in Palbociclib
Yes, Palbociclib is approved by the U.S. Food and Drug Administration (FDA). It was first approved in 2015 for the treatment of advanced or metastatic breast cancer. It is usually given in combination with hormone therapy to help slow the growth of cancer cells.
Another name for Palbociclib is Ibrance, which is its brand name. Ibrance is manufactured by Pfizer and is commonly used to treat certain types of breast cancer. The generic name remains Palbociclib, while Ibrance is used in prescriptions and product labeling.
Palbociclib may form nitrosamines because it is made using certain chemicals like amines and solvents. When these mix with nitrite sources during production or storage, they can create harmful impurities. That’s why regular testing is important to keep the drug safe.
Yes, nitrosamine impurities are closely regulated by health authorities like the FDA and EMA. They have set strict safety limits, and companies must make sure their medicines do not exceed these levels to avoid health risks.
NDSRIs (Nitrosamine Drug Substance-Related Impurities) are specific types of nitrosamines that can form directly from the Palbociclib molecule or its raw materials. These impurities need special attention because they are linked to the drug’s structure.
Yes, Palbociclib can degrade over time and may form nitrosamines under certain conditions. Factors like heat, moisture, and light can break down the drug and lead to chemical reactions that produce these harmful impurities. That’s why proper storage and testing are very important.
High-risk steps include the use of amine-containing chemicals, solvents like DMF or NMP, and any process involving nitrites or strong acids. Also, steps with high heat or oxidizing agents can raise the chance of nitrosamine formation. Careful control during these steps helps reduce the risk.
References
- Xu, X. (2017). Method for preparing palbociclib (U.S. Patent Application No. US20170247380A1). United States Patent and Trademark Office. https://patents.google.com/patent/US20170247380A1
- Control of Nitrosamine Impurities in Human Drugs
- Information about Nitrosamine Impurities in Medications