Introduction: Why Nitrosamine Testing in Vonoprazan Is Important
Nitrosamine testing in Vonoprazan is essential today, especially as global regulations become stricter about harmful impurities in medicines. Vonoprazan is a modern drug used to treat acid-related conditions. But like many medicines, its structure can form nitrosamines, which are unsafe at high levels. This makes testing for these impurities in both its active pharmaceutical ingredient (API) and final product very important.
At ResolveMass Laboratories, we specialize in advanced testing to detect nitrosamines in Vonoprazan. Our services help pharmaceutical companies follow global safety rules while protecting public health.
🔗 Learn more: Nitrosamine Risk Assessment Guide for Your Drug Product
What Is Nitrosamine Testing in Vonoprazan?
Vonoprazan contains chemical groups that can easily react with nitrites and form nitrosamines. These reactions can happen during the drug’s manufacturing, storage, or even due to packaging. Nitrosamines are considered harmful because long-term exposure to them may increase the risk of cancer.
That’s why nitrosamine testing in Vonoprazan has become a must for pharmaceutical companies. At ResolveMass, we follow strict guidelines from the FDA, EMA, and Health Canada to carry out testing. Our labs use advanced equipment like LC-MS/MS and GC-MS to find even the smallest amount of nitrosamines.
Synthesis Process of Vonoprazan
The synthesis of Vonoprazan involves multiple chemical steps, beginning with substituted pyrrole derivatives and building toward the final structure through condensation, reduction, and selective functionalization. Careful control of reaction conditions is needed to prevent the formation of unwanted impurities, including nitrosamines.
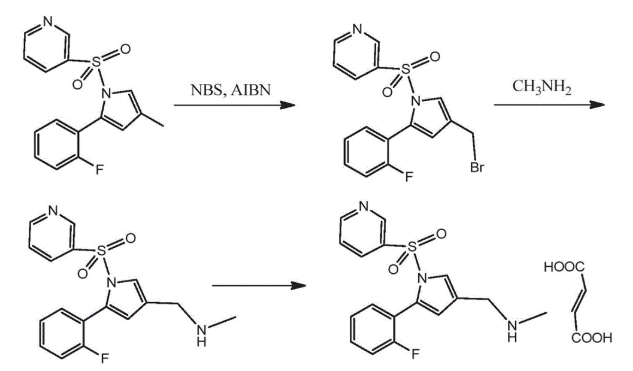
Yu, R., Chen, Y., & Ye, K. (2020). Preparation method of vonoprazan fumarate (Chinese Patent No. CN110452222B). Hangzhou Huadong Medicine Group Biopharmaceutical Co., Ltd.; Hangzhou Zhongmei Huadong Pharmaceutical Co., Ltd. https://worldwide.espacenet.com/patent/search/family/068318682/publication/CN110452222B
Nitrosamine Formation Risks in Vonoprazan API
Vonoprazan contains a secondary amine within its pyrrole ring system, which makes it inherently sVonoprazan contains a secondary amine within its pyrrole ring, making it susceptible to nitrosation under acidic or oxidative conditions—particularly in the presence of sodium nitrite or trace nitrosating agents during synthesis.
The main impurity formed is N-Nitroso Vonoprazan (C₁₇H₁₅FN₄O₃S; MW 374.39), resulting from direct nitrosation of the secondary amine. This is most likely during intermediate stages or final synthetic steps involving nitrite salts or acidic reagents.
Additional nitrosamine impurities reported in patents and impurity databases include:
- N-Nitroso Vonoprazan Impurity 2 (MW ~378.4)
- N-Nitroso Vonoprazan Impurity 3 (MW ~407.4)
These may form depending on structural variations in the API and specific reaction conditions.
To mitigate these risks, manufacturers must:
- Avoid nitrite-containing reagents
- Maintain strict pH control
- Use high-purity solvents
These controls are essential for ensuring product safety and regulatory compliance.
🔗 Explore known NDSRIs: NDSRIs in Nitrosamine Testing
How ResolveMass Manages API Risks
We carefully test each step in the API manufacturing process. Our goal is to detect any nitrosating agents or leftover amines. This way, we can stop nitrosamines from forming early on and help our partners avoid product recalls.
🔗 See detailed risk flow: Nitrosamine CRO Support for Effective Risk Evaluation
Nitrosamine Formation During Vonoprazan Manufacturing (API to Dosage Form)
Vonoprazan contains a secondary amine group within its pyrrole ring, which can easily react with nitrites and form nitrosamines. This reaction can occur during manufacturing, especially when the process involves heat, moisture, or acidic conditions. Under these conditions, and in the presence of small amounts of nitrites from excipients, N-Nitroso Vonoprazan (C₁₇H₁₅FN₄O₃S; molecular weight 374.39) can be formed.
This impurity has been identified during stability testing and was one of the reasons the U.S. FDA issued Complete Response Letters (CRLs) in 2022 and 2023. These regulatory actions required that the level of N-Nitroso Vonoprazan be kept below the acceptable daily intake (ADI) limit. In addition, commercial testing laboratories have reported the presence of other structurally similar nitrosamine impurities with higher molecular weights, although these additional compounds have not been officially named.
To reduce the risk of nitrosamine formation during production, manufacturers should:
- Use excipients with low nitrite content
- Avoid high humidity and acidic processing conditions
- Perform regular testing using validated LC-MS/MS methods to detect N-Nitroso Vonoprazan and related compounds
At ResolveMass, we carry out complete formulation testing. This includes checking for nitrosamine formation at every step — from excipient compatibility to packaging effects.
🧪 We provide targeted Nitrosamine Analysis across all synthetic stages:
ResolveMass Nitrosamine Analysis Lab
Global Regulations for Nitrosamine Testing in Vonoprazan
The U.S. FDA, European Medicines Agency (EMA), and Health Canada have all issued rules about nitrosamines, especially for drugs like Vonoprazan.
Key Requirements by Region
- FDA: Classifies Vonoprazan as high-risk; intake limit of 26.5 ng/day
- EMA: Requires product-specific risk assessments
- Health Canada: Sets intake limits between 18–26 ng/day
Our team helps prepare complete reports for each of these agencies, so your company meets every requirement.
Summary Table – Nitrosamine Risks in Vonoprazan
| Source | Nitrosamine Type | Risk Level |
|---|---|---|
| API Synthesis | N-Methyl-N-nitrosamine | High |
| Storage Degradation | Nitroso-Vonoprazan | Medium |
| Excipients | Nitrite-induced compounds | Variable |
| Solvent Use | DMA, NMP-related nitrosamines | High |
These results are based on real testing and structural analysis done at ResolveMass Laboratories.
🔗 Additional Resources
- Nitrosamine Testing in Apixaban
- Nitrosamine Testing in Sitagliptin
- Nitrosamine Analysis
- Nitrosamine Testing in Amitriptyline
- Nitrosamine Testing in Betahistine
Conclusion: Nitrosamine Testing in Vonoprazan Is a Must
With tighter rules and growing concerns about safety, nitrosamine testing in Vonoprazan is not optional — it’s essential. From raw material testing to finished product analysis, ResolveMass Laboratories ensures your product stays compliant and safe.
Contact us today to learn how we can help protect your product and patients with our trusted nitrosamine testing services.
📞 Contact ResolveMass Laboratories
Need expert guidance?
FAQs: Nitrosamine Testing in Vonoprazan
Vonoprazan contains amine functional groups that can easily react with nitrite-containing substances, especially under acidic or high-temperature conditions. These reactions can lead to the formation of nitrosamines like NDMA and NDEA. Because of its chemical structure, Vonoprazan is more vulnerable to such impurity risks during both synthesis and storage.
Regulatory authorities like the FDA, EMA, Health Canada, and ICH (M7 and Q3D) have made nitrosamine testing mandatory for high-risk APIs such as Vonoprazan. These regulations require manufacturers to conduct thorough risk assessments and implement validated testing protocols. Compliance ensures patient safety and uninterrupted market access.
The most effective methods for nitrosamine detection in Vonoprazan include LC-MS/MS, GC-MS, and headspace GC-MS. These techniques offer high sensitivity and precision, even for very low levels of impurities. The choice of method depends on the dosage form, matrix complexity, and required detection limits.
Yes, excipients used in Vonoprazan formulations can react with amine groups to form nitrosamines, especially if they contain or release nitrites. Under certain storage or processing conditions, these reactions may become more likely. That’s why full formulation-level risk assessments are necessary during development.
The acceptable daily intake (ADI) for nitrosamines varies based on the specific compound, generally ranging between 18 ng/day and 96 ng/day. Regulatory bodies like the FDA and EMA define these limits to ensure patient safety. Exceeding these limits can lead to recalls or halted product approvals.
Risk can be minimized by using high-purity raw materials, avoiding nitrosating agents, and controlling reaction parameters such as pH and temperature. Stability studies under stress conditions also help identify potential nitrosamine formation. Optimizing the synthesis and packaging processes further reduces risks.
Nitrosamine testing at ResolveMass typically takes 10–15 business days, depending on the sample type and complexity. This timeline includes method development, validation, and detailed reporting. We also offer expedited services if faster turnaround is needed for regulatory submissions or batch release.
It is highly recommended to perform batch-wise testing, especially for commercial and clinical batches. This ensures that each lot meets regulatory safety standards and does not exceed nitrosamine limits. Regular testing also supports continuous product quality and patient trust.
Yes, we specialize in developing and validating custom test methods tailored to your Vonoprazan formulation. Whether it’s a tablet, suspension, or modified-release form, we adapt our approach based on matrix type, sensitivity needs, and regulatory expectations. This ensures precision and full compliance.
References
- Yu, R., Chen, Y., & Ye, K. (2020). Preparation method of vonoprazan fumarate (Chinese Patent No. CN110452222B). Hangzhou Huadong Medicine Group Biopharmaceutical Co., Ltd.; Hangzhou Zhongmei Huadong Pharmaceutical Co., Ltd. https://worldwide.espacenet.com/patent/search/family/068318682/publication/CN110452222B
- Control of Nitrosamine Impurities in Human Drugs


