Introduction
Selecting the right nitrosamine testing lab in Canada is crucial for pharmaceutical manufacturers aiming to comply with regulatory standards and ensure product safety. With the increased regulatory scrutiny from Health Canada, the U.S. FDA, and the European Medicines Agency (EMA), nitrosamine impurity testing has become a mandatory step in pharmaceutical development and production. Choosing a qualified laboratory is more than just a regulatory checkbox—it’s a strategic decision that impacts your timeline, product integrity, and market approval.
In this comprehensive guide, we’ll walk you through the essential factors to consider when selecting a nitrosamine testing lab in Canada for your API (Active Pharmaceutical Ingredient) or drug product. We’ll also highlight the role of validated methods, technology, compliance, turnaround times, and customer support.
Why Nitrosamine Testing Lab in Canada is Critical in Pharma Projects
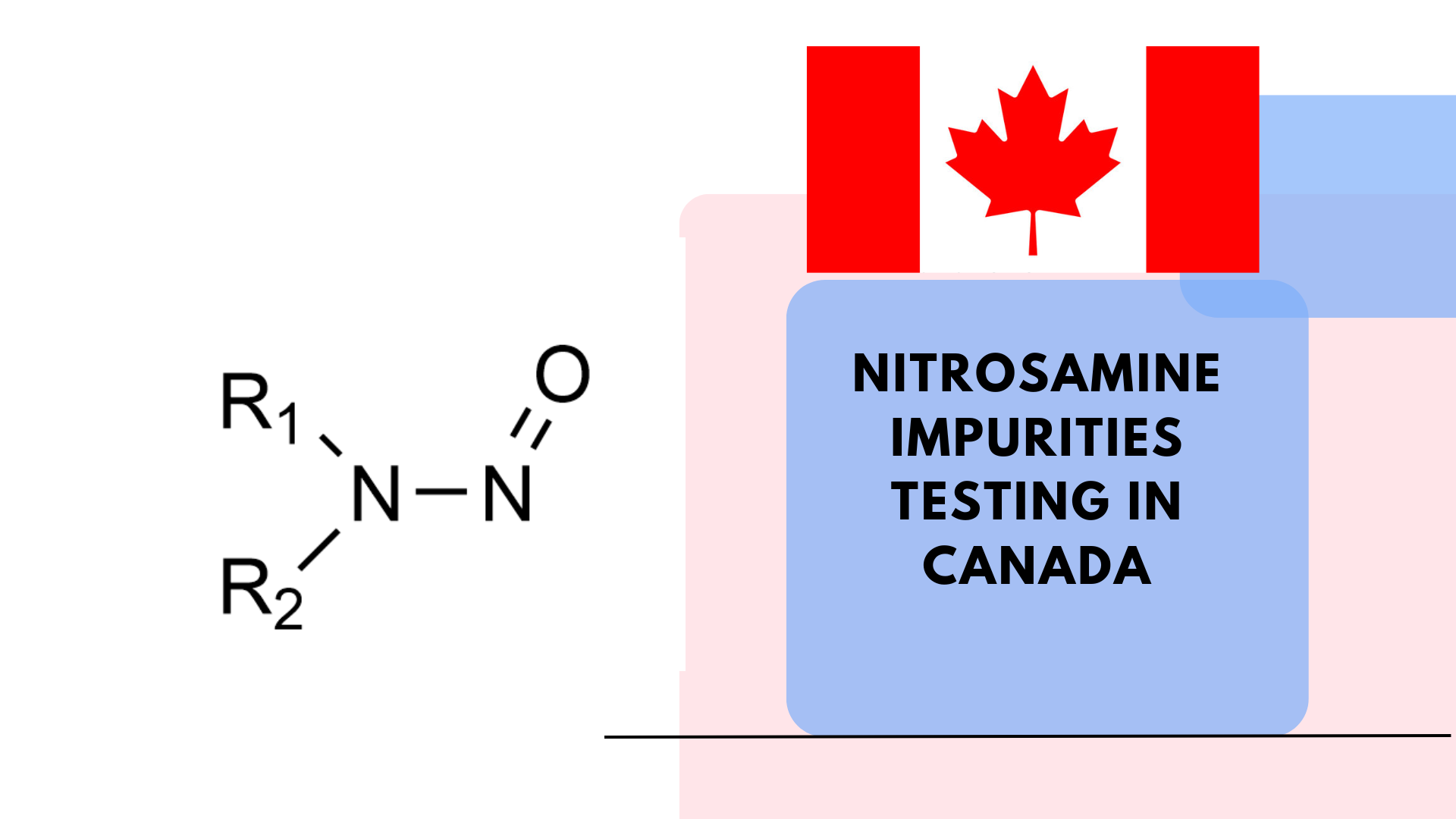
Nitrosamines are a group of potent genotoxic impurities known to pose carcinogenic risks to humans [1]. The detection and quantification of nitrosamines in APIs and drug products is not only necessary for consumer safety but also mandated by regulatory bodies globally.
- Health Canada Guidance: Requires a risk-based approach and confirmatory testing to determine nitrosamine levels.
- ICH M7 Guidelines: Focuses on controlling mutagenic impurities in pharmaceuticals [2].
- FDA Recall Alerts: Demonstrates the importance of routine nitrosamine testing to prevent costly and reputation-damaging product recalls [3].
Partnering with a certified and technologically advanced nitrosamine testing lab in Canada helps you meet these expectations and maintain the integrity of your pharma project.
Explore our Nitrosamine Analysis Services
Key Factors in Selecting a Nitrosamine Testing Lab in Canada
1. Regulatory Compliance & Accreditation for Nitrosamine Testing Lab in Canada
Ensure that the lab complies with:
- Health Canada and FDA standards
- ICH M7, Q3D, and Q3C guidelines
- Certifications such as ISO/IEC 17025, GLP, and cGMP
A reputable nitrosamine testing lab in Canada should be able to demonstrate compliance with international standards and provide quality certificates upon request.
2. Advanced Analytical Technologies
The best labs offer cutting-edge instrumentation for nitrosamine impurity testing, including:
- LC-MS/MS (Liquid Chromatography – Tandem Mass Spectrometry) for detecting trace levels of nitrosamines [4]
- GC-MS for volatile nitrosamine analysis
- HRMS (High-Resolution Mass Spectrometry) for precise identification and quantification
- Isotope-Dilution Techniques for maximum accuracy
ResolveMass Laboratories uses all these validated platforms to ensure robust results and meet regulatory thresholds.
Visit Our Nitrosamine Testing Services
3. Testing Capabilities Across Drug Development Phases
Your chosen lab must support:
- Early-stage development (screening for potential nitrosamine precursors)
- Pilot batches and process validation
- Post-marketing surveillance (stability & shelf-life studies)
- Packaging interaction assessments
A full-service nitrosamine testing lab in Canada will support your project from formulation to final release.
4. Custom Method Development and Validation for Nitrosamine Testing Lab in Canada
Each drug formulation is unique. A good lab will:
- Develop customized methods based on your matrix and risk profile
- Perform ICH-compliant method validation (accuracy, precision, LOD, LOQ, specificity)
Custom solutions are especially important for complex APIs or biologics with high-risk precursors.
Every pharmaceutical product has a unique chemical composition, and nitrosamine testing must be customized accordingly. A qualified nitrosamine lab in Canada should provide:
- Custom Method Development: Tailoring analytical methods based on the specific API or drug formulation, synthetic route, and risk profile. This includes selection of proper extraction techniques, column chemistries, and ionization modes.
- Matrix-Specific Sensitivity: Optimizing methods to achieve detection limits at or below the regulatory threshold (typically <30 ppb).
- Validation of Analytical Methods: Ensuring the accuracy, precision, specificity, linearity, robustness, and limit of detection (LOD) are in line with ICH Q2(R1) and Health Canada guidelines [2].
- Robust Documentation: Detailed method validation protocols and reports suitable for regulatory submissions and audits.
This tailored approach ensures the test method is both scientifically sound and fit for regulatory acceptance, offering greater confidence for your drug development lifecycle.
Why Mass Spectrometry Expertise is Necessary in Nitrosamine Testing lab in Canada
Mass spectrometry (MS) expertise is essential for accurate and reliable nitrosamine testing lab in Canada operations. Due to the ultra-trace levels at which nitrosamines must be detected—often in the parts-per-billion (ppb) range—mass spectrometry provides the sensitivity and specificity required for regulatory compliance.
- High Sensitivity: Techniques like LC-MS/MS and GC-MS allow for the detection of nitrosamines even at concentrations below regulatory limits, reducing the risk of false negatives and ensuring patient safety.
- High Selectivity: MS methods can differentiate nitrosamines from other structurally similar compounds, avoiding cross-reactivity and improving data integrity.
- Complex Matrices: MS is essential when analyzing nitrosamines in complex pharmaceutical formulations, where interferences from excipients can mask or distort results.
- Structural Elucidation: High-resolution MS and tandem MS techniques are useful for identifying unknown nitrosamine compounds or degradation products.
- Regulatory Acceptance: Health Canada, FDA, and EMA all require validated MS-based methods for confirmatory nitrosamine impurity testing, making it the gold standard in analytical chemistry for this purpose [3][4].
Having in-house MS expertise ensures method development, validation, troubleshooting, and interpretation are carried out with precision and efficiency, which is crucial for both R&D and commercial phases of pharmaceutical production.
5. Fast Turnaround Time and Scalability for Nitrosamine Testing Lab in Canada
In the pharma industry, speed matters. Look for a nitrosamine testing lab in Canada that offers:
- Expedited analysis for high-priority samples
- Scalable capacity to accommodate batch releases
- Real-time data reporting through client portals
At ResolveMass, we understand the need for fast, reliable testing. Our processes are optimized for efficiency without compromising data integrity.
6. Expert Consultation and Regulatory Support for Nitrosamine Testing Lab in Canada
A good lab does more than just run tests. Choose a partner who:
- Assists with nitrosamine risk assessments
- Supports regulatory filings (e.g., ANDA, NDA, CTA)
- Advises on mitigation strategies and root cause analysis
ResolveMass provides technical reports and scientific consultation with every testing project.
7. Proven Experience with Nitrosamine Testing Lab in Canada
Experience matters. Ask your lab:
- How many nitrosamine projects have they completed?
- Do they have experience with your drug class or compound type?
- Can they share anonymized case studies?
Our team at ResolveMass has worked with Canadian and international pharmaceutical companies, supporting everything from generics to biosimilars.
Why Choose ResolveMass as Your Nitrosamine Testing Lab in Canada?
ResolveMass Laboratories is a trusted nitrosamine testing lab in Canada offering:
- Comprehensive testing solutions for APIs, excipients, finished drug products, and packaging materials
- State-of-the-art instrumentation with ultra-sensitive detection limits
- Compliance with global regulatory standards (Health Canada, FDA, EMA)
- Quick turnaround times and transparent reporting
- Technical consultation and project guidance
Whether you’re facing a regulatory deadline, launching a new drug, or simply staying ahead of compliance, ResolveMass is your partner for reliable nitrosamine analysis.
Discover our Nitrosamine Testing Solutions
Conclusion
Choosing the right nitrosamine testing lab in Canada is vital for protecting patient health, avoiding regulatory action, and ensuring your pharma product is safe for market. From equipment and method validation to expert consultation and regulatory support, every factor counts.
When you choose ResolveMass, you’re investing in quality, speed, and scientific excellence. Contact us today to get started.
References
- EMA. (2021). Assessment and Mitigation of Nitrosamine Risk in Human Medicines. https://www.ema.europa.eu/en/documents/referral/nitrosamines-emea-h-a53-1490-assessment-report_en.pdf
- FDA. (2021). Control of Nitrosamine Impurities in Human Drugs. https://www.fda.gov/media/141720/download
- Health Canada. (2020). Guidance on Nitrosamine Impurities in Medications. https://www.canada.ca/en/health-canada/services/drugs-health-products.html
- ICH. (2023). ICH M7(R2) – Control of Mutagenic Impurities. https://database.ich.org/sites/default/files/M7_R2_Guideline_Step4_2023_0223.pdf