Introduction
For emerging biotech companies, deciding whether to outsource bioanalysis for biotech startups or invest in in-house laboratory infrastructure is a critical strategic decision. Bioanalysis is a cornerstone of drug discovery, preclinical development, and clinical trials—but it is also capital-intensive, technically complex, and highly regulated.
In this article, we explore when biotech startups should outsource bioanalysis instead of building in-house labs, helping founders, CSOs, and investors make informed, scalable, and regulatory-ready decisions.
Summary
- Outsourcing bioanalysis helps biotech startups reduce capital expenditure and operational risk.
- Early drug discovery and preclinical stages benefit most from outsourced bioanalytical services.
- Regulatory-compliant bioanalysis is easier to achieve through experienced CRO partners.
- Building in-house labs makes sense only after pipeline maturity and funding stability.
- Many biotech startups outsource bioanalysis to accelerate timelines and attract investors.
1: What Is Bioanalysis and Why Is It Critical for Biotech Startups?
Bioanalysis is the scientific discipline focused on the quantitative measurement of drugs, metabolites, biomarkers, and biologics in biological matrices such as plasma, serum, whole blood, urine, tissue, or cerebrospinal fluid. It forms the analytical backbone of drug discovery and development by generating reliable data that supports scientific decision-making and regulatory submissions.
Modern bioanalysis encompasses a wide range of activities, including:
- Bioanalytical quantification of small and large molecules
- LC-MS/MS bioanalysis of xenobiotics
- Large molecule bioanalysis using ligand-binding assays
- Cell and gene therapy bioanalysis for advanced modalities
You can explore the full scope of these capabilities through ResolveMass’s comprehensive bioanalytical services overview.
Core Areas Supported by Bioanalysis
Bioanalysis directly supports:
- Pharmacokinetics (PK): Understanding absorption, distribution, metabolism, and excretion through specialized PK/PD bioanalysis
- Pharmacodynamics (PD): Correlating exposure with biological response
- Toxicokinetics (TK): Measuring systemic exposure in safety studies
- Biomarker analysis: Evaluating target engagement and disease progression
For biotech startups, bioanalysis underpins nearly every early milestone. Without robust bioanalytical data, advancing from discovery into regulated development becomes extremely difficult.
To understand the foundational importance further, see:
👉 https://resolvemass.ca/why-is-bioanalysis-important/
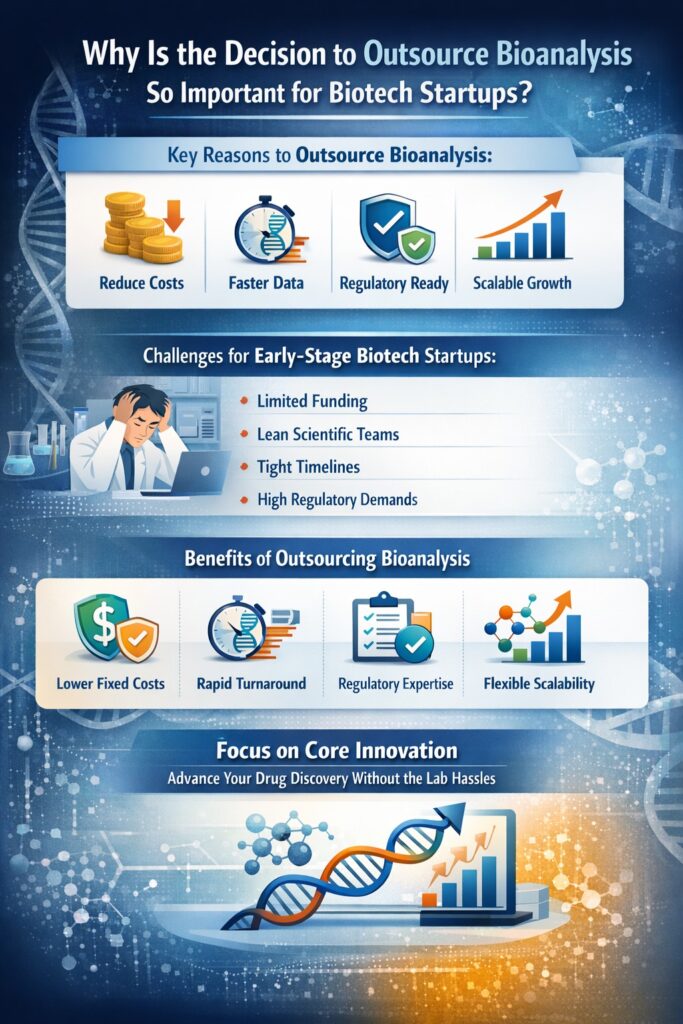
2: Why Is the Decision to Outsource Bioanalysis So Important for Biotech Startups?
The decision to outsource bioanalysis for biotech startups is strategically important because it directly influences cost efficiency, development timelines, regulatory success, and long-term business scalability. For early-stage biotech companies, this choice often determines how quickly and reliably a program can move from discovery to regulated development.
Early-stage biotech startups typically operate under significant constraints, including limited funding, lean scientific teams, and aggressive milestones driven by investor expectations. In this environment, building and maintaining internal bioanalytical laboratory services can become a major operational burden rather than a strategic advantage.
2.1 Impact on Cost Structure
Outsourcing bioanalysis allows biotech startups to convert large fixed costs into predictable, project-based expenses. Establishing an in-house bioanalytical lab requires:
- Significant capital investment in LC-MS/MS instruments and analytical platforms
- Ongoing maintenance, calibration, and software validation costs
- Hiring and retaining experienced bioanalytical scientists
- Continuous investment in quality systems and audits
By choosing to outsource bioanalysis for biotech startups, companies avoid these upfront and recurring expenses, preserving cash runway and allocating capital toward core research andure.
2.2 Impact on Speed to Data
Speed-to-data is critical for biotech startups competing for funding, partnerships, and regulatory milestones. Outsourced bioanalytical partners operate with:
- Established analytical workflows
- Pre-validated methods
- Dedicated bioanalytical teams
This enables faster study initiation, shorter turnaround times, and quicker scientific decision-making compared to building internal capabilities from scratch.
2.3 Impact on Regulatory Readiness
Regulatory compliance is one of the most complex challenges for early-stage biotech companies. Bioanalytical data must meet strict regulatory standards for IND and CTA submissions. Outsourcing to an experienced CRO ensures:
- Compliance with FDA, EMA, and ICH bioanalytical guidelines
- Audit-ready documentation and validated processes
- Reduced risk of regulatory delays or data rejection
For startups without in-house regulatory infrastructure, outsourcing significantly lowers compliance risk.
2.4 Impact on Long-Term Scalability
Outsourcing bioanalysis provides startups with the flexibility to scale analytical support as pipelines evolve. As programs advance or change direction, outsourced services can be expanded, reduced, or redirected without the constraints of fixed laboratory infrastructure or staffing commitments.
This scalability allows leadership teams to remain focused on scientific innovation, strategic partnerships, and clinical advancement rather than laboratory operations.
2.5 Strategic Focus on Core Innovation
Most importantly, outsourcing bioanalysis enables biotech startup leadership to concentrate on what matters most—advancing novel therapeutics. Instead of managing laboratory buildouts, regulatory audits, and staffing challenges, teams can focus on:
- Target validation and biology
- Lead optimization
- Clinical development strategy
- Investor and partner engagement
In summary, the decision to outsource bioanalysis for biotech startups is not simply an operational choice—it is a strategic one that directly impacts a company’s ability to innovate, comply, scale, and succeed.
Outsourcing allows startups to immediately access regulated bioanalytical services without building infrastructure from scratch.
3: When Should Biotech Startups Outsource Bioanalysis Instead of Building In-House Labs?
Biotech startups should outsource bioanalysis when speed, compliance, and cost-efficiency outweigh the need for full internal control.
Outsourcing is particularly advantageous when:
- Programs are in discovery or preclinical phases
- Sample volumes fluctuate
- Methods are still evolving
- Regulatory submissions (IND/CTA) are approaching
ResolveMass supports these stages through bioanalytical services in drug development and IND/NDA-enabling bioanalysis.
4: Stage of Company: Early Discovery and Preclinical Development
Outsourcing bioanalysis for biotech startups is most effective during early discovery and preclinical development.
At this stage:
- Assays change frequently
- Compound portfolios evolve
- Flexibility matters more than ownership
Outsourced partners can rapidly support:
- Discovery vs regulated bioanalysis transitions
- High-throughput bioanalysis for screening studies
Key Advantages
- Easy scalability
- No upfront investment in LC-MS/MS platforms
- Access to validated bioanalytical method development
- Faster PK, TK, and biomarker turnaround
5: Cost Considerations: In-House Labs vs Outsourced Bioanalysis
Cost is often the single most decisive factor when biotech startups choose to outsource bioanalysis.
In-House Bioanalytical Lab Cost Components
Building and maintaining an internal lab requires substantial and recurring investment, including:
- LC-MS/MS instruments, software, and ongoing maintenance
- Laboratory space, utilities, and environmental controls
- Hiring and retaining qualified bioanalytical scientists
- Method validation and matrix-effect assessments
- Method development, validation, and documentation
- QA systems, audits, and regulatory inspections
Outsourced Bioanalysis Cost Advantages
Outsourcing offers a leaner, more predictable financial model:
- Project-based or pay-per-study pricing
- No equipment depreciation or maintenance costs
- No recruitment, training, or retention burden
- Transparent and investor-friendly budgeting
| Cost Factor | In-House Lab | Outsourced Bioanalysis |
|---|---|---|
| Capital Investment | Very High | None |
| Regulatory Setup | Complex | Included |
| Staffing | Full-Time | On-Demand |
| Scalability | Limited | High |
Startups often choose affordable bioanalytical services for biotech startups to preserve runway.
For cost transparency, see:
👉 https://resolvemass.ca/bioanalytical-testing-services-cost/
6: Regulatory and Compliance Challenges for In-House Bioanalysis
Regulatory compliance is one of the strongest drivers for outsourcing bioanalysis for biotech startups.
Bioanalytical studies must comply with:
- FDA Bioanalytical Method Validation (BMV) Guidelines
- EMA and ICH regulatory frameworks
- GLP requirements for preclinical studies
Establishing compliance in-house requires:
- Comprehensive SOP development
- Dedicated QA/QC infrastructure
- Continuous audit readiness
- Deep regulatory expertise
Achieving this internally requires validated bioanalytical method validation and audit-ready systems.
Experienced CROs already maintain compliance across clinical bioanalytical services and regulated studies.
Experienced CROs like ResolveMass Laboratories Inc. already operate within validated, inspection-ready environments, significantly reducing regulatory risk and submission delays.
7: Speed-to-Data: Why Outsourcing Accelerates Drug Development
Outsourcing bioanalysis enables biotech startups to generate high-quality data faster than building labs from scratch.
Key speed advantages include:
- Established analytical workflows
- Pre-validated platforms and methods
- Established LC-MS/MS workflows
- Expertise in small molecule vs large molecule bioanalysis
- Parallel method development and sample analysis
- Rapid study initiation and execution
Speed-to-data directly improves investor confidence and partnering outcomes.
In competitive funding and partnering environments, faster access to reliable data often translates directly into stronger valuations and accelerated development milestones.
8: Talent and Expertise Gaps in Early-Stage Biotech Companies
Recruiting experienced bioanalytical scientists is both difficult and costly for early-stage startups.
Common challenges include:
- Limited availability of senior LC-MS/MS experts
- High salary and retention costs
- Significant onboarding and training time
By choosing to outsource bioanalysis for biotech startups, companies gain immediate access to multidisciplinary teams with:
- Proven method development and validation expertise
- Experience across small molecules, biologics, and biomarkers
- Deep understanding of regulatory expectations
9: When Does It Make Sense to Build an In-House Bioanalytical Lab?
Building an in-house bioanalytical lab typically makes sense only once a startup reaches operational maturity.
Key indicators include:
- Multiple assets in late preclinical or clinical stages
- Stable, long-term funding
- High and consistent sample volumes
- Ongoing need for internal testing
Even at this stage, many companies adopt a hybrid model, continuing to outsource complex, regulated, or specialized bioanalysis.
Even then, many adopt a hybrid model and continue bioanalytical outsourcing for specialized studies.
10: Outsourcing Bioanalysis as a Strategic Investor Signal
Investors often view outsourced bioanalysis as a sign of strong operational discipline and strategic focus.
Why investors favor outsourcing:
- Lower burn rate and financial risk
- Reduced regulatory exposure
- Access to validated, decision-ready data
- Greater focus on scientific and clinical innovation
- Control burn rate
- Reduce regulatory risk
- Use experienced bioanalytical CROs
👉 https://resolvemass.ca/bioanalytical-cro-for-drug-discovery/
Startups that outsource bioanalysis effectively often progress more smoothly through funding rounds and partnerships.
11: How to Choose the Right Bioanalytical CRO Partner
Selecting the right CRO is critical when you outsource bioanalysis for biotech startups.
Key evaluation criteria include:
- Proven regulatory inspection track record
- Advanced LC-MS/MS and biomarker capabilities
- Strong data integrity and compliance culture
- Clear, transparent scientific communication
- Flexible and startup-friendly engagement models
- North American regulatory experience
ResolveMass Laboratories Inc. specializes in supporting biotech startups with scalable, compliant, and high-quality bioanalytical services across discovery, preclinical, and clinical development.
ResolveMass provides end-to-end bioanalytical services and laboratory services tailored to startups.
12: Future Trends: Outsourcing Bioanalysis in the Biotech Startup Ecosystem
The trend to outsource bioanalysis for biotech startups continues to accelerate globally.
Key drivers include:
- Increasing regulatory complexity
- Rapid advances in analytical technologies
- Demand for faster decision-making
- Advanced modalities like cell and gene therapies
- Growing emphasis on capital efficiency
Outsourcing bioanalysis is no longer just a cost-saving measure—it is a strategic growth enabler.
Conclusion
In today’s competitive biotech landscape, outsourcing bioanalysis for biotech startups is often the most efficient, compliant, and scalable approach—particularly during early discovery and preclinical development. Building an in-house bioanalytical lab too early can strain resources, slow progress, and increase regulatory risk.
By partnering with an experienced bioanalytical CRO like ResolveMass Laboratories Inc., biotech startups can stay focused on innovation while ensuring their data meets the highest scientific and regulatory standards.
Frequently Asked Questions:
In-house development means tasks, projects, or systems are designed, built, and maintained by an organization’s own internal employees using internal resources.
Outsourcing means contracting external vendors or service providers to perform specific tasks or develop systems on behalf of the organization.
Key difference:
-In-house development offers greater control and direct oversight.
-Outsourcing provides access to external expertise, lower costs, and faster scalability.
No, there will not necessarily be less need for in-house systems analysts.
Instead, their role is evolving.
As companies outsource development:
-In-house systems analysts shift from building systems to managing requirements, vendors, integration, and quality oversight.
-They act as strategic liaisons between business stakeholders and outsourcing partners.
Outsourcing reduces hands-on development but increases the need for analysts who can define requirements, ensure alignment, and manage vendor performance.
Benefits of In-House Development:
-Full control over processes and data
-Deep organizational knowledge
-Strong alignment with internal goals
-Better protection of sensitive information
Benefits of Outsourcing:
-Lower operational and capital costs
-Access to specialized expertise
-Faster project delivery
-Greater flexibility and scalability
-Organizations often use a hybrid model to combine the strengths of both.
The four major advantages of outsourcing are:
~Cost Reduction – Lower labor, infrastructure, and operational costs
~Access to Expertise – Specialized skills not available internally
~Faster Time-to-Market – Accelerated project execution
~Scalability & Flexibility – Easily scale resources up or down as needed
The two fundamental reasons for outsourcing are:
~Cost Efficiency – Reducing expenses related to staffing, infrastructure, and training
~Strategic Focus – Allowing organizations to focus on core competencies while external experts handle non-core or specialized tasks
Reference
- Stephen Lowes.Outsourcing in Bioanalysis: A CRO Perspective.https://www.tandfonline.com/doi/full/10.4155/bio-2017-4994
- Tom Verhaeghe.Bioanalytical Outsourcing Strategy at Janssen Research and Development.https://www.tandfonline.com/doi/abs/10.4155/bio.14.105
- Yan Song,Raj Dhodda,Jun Zhang &Jens Sydor.A High Efficiency, High Quality and Low Cost Internal Regulated Bioanalytical Laboratory to Support Drug Development Needs.https://www.tandfonline.com/doi/abs/10.4155/bio.14.104
- Dieter Zimmer.Bioanalytical Outsourcing Models in Pharmaceutical Companies.https://www.tandfonline.com/doi/full/10.4155/bio-2017-4995
- Roger Hayes.Bioanalytical Outsourcing: Transitioning from Pharma to Cro.https://www.tandfonline.com/doi/full/10.4155/bio-2017-4996
- Yue-Ting WangORCID Icon,Estelle M Maes,Lance Heinle,Kenneth Ruterbories,Stella Doktor,Mary Larsen,Amanda Olson,Andrew Lee,Cecilia Van Handel,Qin C Ji,Kelly Desino &Gary J Jenkins.Integrity and Efficiency: AbbVie’s Journey of Building an Integrated Nonregulated Bioanalytical Laboratory.https://www.tandfonline.com/doi/abs/10.4155/bio-2023-0012

