Introduction
Outsourced bioanalysis for drug development has become a foundational strategy for lean biotech and pharmaceutical teams seeking to advance programs efficiently without sacrificing scientific rigor. As drug development grows more complex, data-driven, and regulated, maintaining in-house bioanalytical infrastructure is often impractical for early-stage and resource-constrained organizations. Many companies instead rely on specialized partners offering end-to-end bioanalytical services in drug development and a comprehensive bioanalytical services overview.
Lean development teams must generate high-quality, regulator-ready bioanalytical data while operating with limited headcount, capital, and time. Outsourcing bioanalysis to an experienced bioanalytical CRO like ResolveMass Laboratories Inc. enables teams to meet these demands while staying focused on innovation, clinical strategy, and value creation through cost-effective bioanalytical services.
Summary
- Outsourced bioanalysis for drug development enables lean teams to access advanced analytical expertise without building internal labs.
- Strategic outsourcing reduces timelines, controls costs, and minimizes regulatory risk.
- Expert bioanalytical partners provide validated methods, regulatory-ready data, and scalable support across drug development stages.
- Lean biotech and virtual pharma teams rely on bioanalytical outsourcing to remain focused on core R&D.
- Choosing the right CRO transforms bioanalysis from a cost center into a strategic advantage.
1: What Is Outsourced Bioanalysis for Drug Development?
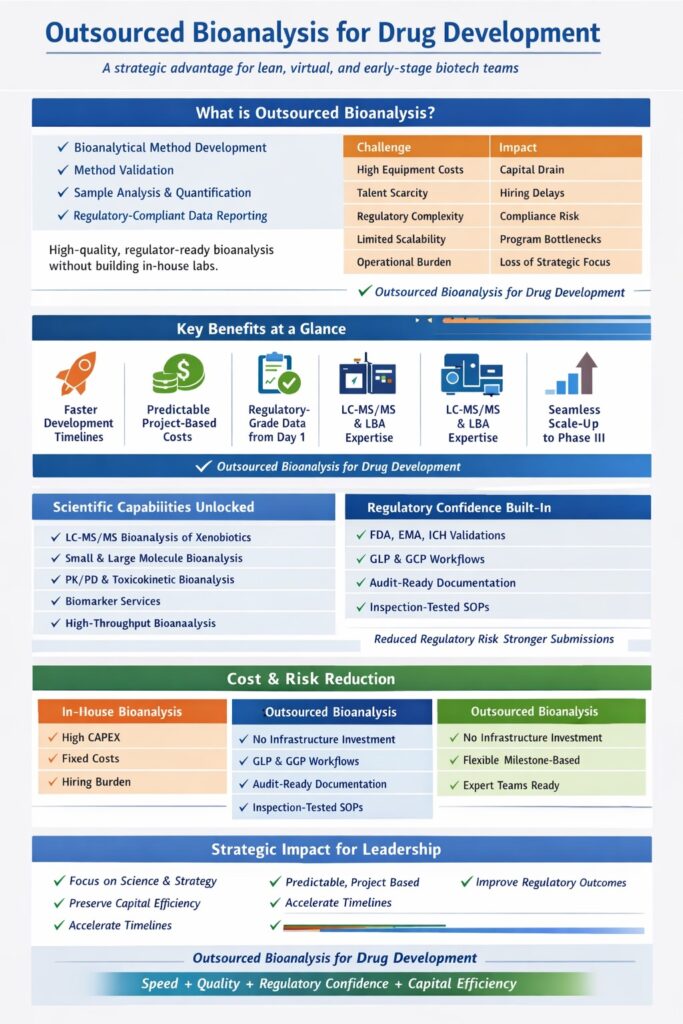
Outsourced bioanalysis for drug development refers to the strategic practice of partnering with a specialized contract research organization (CRO) to manage bioanalytical activities required across preclinical and clinical development. These activities include bioanalytical method development, method validation, sample analysis, data reporting, and regulatory documentation.
Outsourced bioanalysis for drug development refers to partnering with a specialized contract research organization to perform bioanalytical testing, bioanalytical method development, bioanalytical method validation, sample analysis, bioanalytical quantification, and regulatory documentation across preclinical and clinical stages.
In practical terms, outsourced bioanalysis allows drug developers to leverage highly specialized analytical infrastructure and scientific expertise without building and maintaining internal laboratories. This model is particularly valuable for lean, virtual, and early-stage biotech companies that must balance scientific rigor with speed, cost control, and regulatory compliance.
Through outsourced bioanalysis for drug development, organizations gain access to:
- Advanced LC-MS/MS bioanalytical services including LC-MS/MS bioanalysis of xenobiotics
- Expertise in small molecule vs large molecule bioanalysis and large molecule bioanalysis
- Scalable bioanalytical services for small and large molecule quantification
For lean drug development teams, outsourcing bioanalysis is not simply an operational convenience—it is a strategic approach that enables scientific excellence while preserving organizational agility.
2: Why Lean Drug Development Teams Cannot Rely on In-House Bioanalysis
Lean drug development teams struggle with in-house bioanalysis due to cost, complexity, and regulatory burden. Building and maintaining compliant bioanalytical laboratory services requires significant investment in infrastructure, personnel, and quality systems.
In-house teams often face challenges related to bioanalytical matrix effects, evolving regulatory expectations, and the transition from discovery vs regulated bioanalysis.
By leveraging bioanalytical services outsourcing for pharma, lean teams eliminate these risks while ensuring scientific and regulatory rigor.
Key Challenges of In-House Bioanalysis
| Challenge | Impact on Lean Teams |
|---|---|
| Capital-intensive equipment | Requires significant upfront investment in LC-MS/MS systems, automation, and facilities |
| Specialized scientific talent | Difficult and costly to recruit, train, and retain experienced bioanalytical scientists |
| Regulatory compliance burden | Increases risk of audit findings, inspection delays, and rework |
| Limited scalability | Internal labs struggle to handle fluctuating sample volumes during clinical expansion |
| Operational distraction | Diverts leadership focus from drug discovery, clinical strategy, and fundraising |
Outsourced bioanalysis for drug development removes these barriers by shifting responsibility to organizations whose sole focus is bioanalytical science. This enables lean teams to maintain flexibility while ensuring their data meets the highest scientific and regulatory standards.
3: How Outsourced Bioanalysis Accelerates Drug Development Timelines
Outsourced bioanalysis for drug development accelerates timelines by providing immediate access to established workflows, validated platforms, and experienced scientific teams. Rather than spending months building internal capability, companies can initiate bioanalytical work almost immediately.
From IND-enabling studies through late-phase clinical trials, speed is critical—but speed without data integrity is meaningless. Specialized CRO partners such as ResolveMass Laboratories Inc. operate with:
- Established SOPs and validated analytical platforms, reducing method development and qualification timelines
- Proven sample logistics and data pipelines, ensuring efficient handling of large clinical sample sets
- Parallel processing capabilities, allowing multiple studies or programs to progress simultaneously
By outsourcing bioanalysis, lean teams eliminate bottlenecks and gain predictable turnaround times without compromising regulatory readiness or data quality.
ResolveMass supports rapid execution through:
- PK/PD bioanalysis and toxicokinetic bioanalysis
- Efficient clinical bioanalytical services
- High-throughput bioanalysis for large clinical sample sets
This enables lean teams to meet aggressive timelines without compromising data quality.
4: Why Bioanalytical Expertise Is Central to Regulatory Success
Bioanalysis plays a direct role in regulatory decision-making, influencing everything from dose selection to clinical progression and approval outcomes. Regulatory agencies expect bioanalytical data to be scientifically sound, reproducible, and fully traceable.
Bioanalysis directly impacts regulatory confidence, dose justification, and clinical progression. Outsourced bioanalysis for drug development ensures alignment with global regulatory expectations through regulated bioanalytical services and GLP bioanalytical services.
Outsourced bioanalysis for drug development ensures that data generation is aligned with regulatory expectations from the very beginning of a program.
Key regulatory advantages include:
- FDA, EMA, and ICH-aligned validation strategies tailored to development stage and molecule type
- Experienced handling of audits and inspections, minimizing regulatory risk
- Scientifically justified method development decisions, supporting defensible data interpretation
- Robust documentation and data traceability, critical for submissions and regulatory responses
Lean teams benefit enormously from CRO partners who possess deep institutional knowledge of global regulatory expectations and evolving bioanalytical guidance.
5: How Outsourced Bioanalysis Reduces Cost and Financial Risk
Outsourced bioanalysis for drug development converts fixed infrastructure costs into predictable project-based expenses. This is critical for startups relying on affordable bioanalytical services for biotech startups and evaluating bioanalytical testing services cost.
Instead of investing heavily in infrastructure, lean teams gain:
- Transparent, milestone-based pricing, aligned with program progression
- No equipment maintenance, depreciation, or upgrade costs
- Flexible scale-up or scale-down, depending on study phase and sample volume
- Lower long-term financial exposure, especially if programs pivot or terminate
This cost efficiency is particularly valuable for seed-stage, Series A, and virtual biotech companies where capital preservation directly impacts survival and success.
ResolveMass supports submissions through bioanalytical services for IND/NDA submissions and helps teams navigate complex validation and inspection requirements.
6: The Strategic Advantage of Focus: Let Scientists Focus on Science
Successful lean drug development teams focus on high-impact scientific and strategic decisions rather than operational complexity. Managing in-house bioanalysis often pulls leadership and scientists into logistical, staffing, and compliance challenges.
By outsourcing bioanalysis, internal teams remain focused on high-value activities such as molecular design, translational strategy, and fundraising. This approach is especially important for virtual biotech companies, which rely on bioanalytical CROs for virtual biotech and structured virtual bioanalytical strategies.
Outsourcing bioanalysis allows teams to:
- Concentrate on molecular design, translational strategy, and clinical development planning
- Allocate internal resources toward intellectual property, partnerships, and fundraising
- Avoid micromanaging analytical execution and troubleshooting
- Make faster go/no-go decisions based on reliable, high-quality data
Outsourced bioanalysis for drug development empowers leadership teams to operate strategically, using data as a decision-making tool rather than an operational burden.
7: Why Outsourced Bioanalysis Is Essential Across Development Stages
Outsourced bioanalysis for drug development provides continuity and consistency throughout the entire drug development lifecycle.
Preclinical Development
- Pharmacokinetic and toxicokinetic analysis
- Biomarker assay development and feasibility studies
- Early method optimization to support IND-enabling work
Clinical Development
- GLP- and GCP-compliant bioanalysis
- Sample analysis across Phase I, II, and III trials
- Cross-study method transfer and analytical consistency
- Bioanalytical CRO services for PK and TK
Late-Stage and Post-Approval
- Method lifecycle management
- Regulatory support
- Biosimilar bioanalysis and cell and gene therapy bioanalysis
A trusted CRO partner ensures scientific memory is preserved across phases, reducing variability and risk as programs mature.
8: How ResolveMass Laboratories Inc. Delivers Trustworthy Outsourced Bioanalysis
ResolveMass Laboratories Inc. delivers outsourced bioanalysis for drug development with a strong emphasis on scientific rigor, regulatory alignment, and strategic collaboration.
ResolveMass delivers outsourced bioanalysis for drug development with scientific depth, regulatory expertise, and transparent collaboration. Its services span bioanalytical services in North America and support discovery-stage programs through bioanalytical CROs for drug discovery.
Key differentiators include:
- Senior scientist-led project oversight, ensuring informed decision-making
- Fit-for-purpose method development, aligned with program goals
- Transparent communication and proactive problem-solving
- Proven experience across small molecules and complex modalities
- Uncompromising commitment to data integrity and quality
ResolveMass operates as an extension of your internal team—providing insight, not just data.
9: Key Considerations When Choosing an Outsourced Bioanalytical Partner
Selecting the right CRO is critical to the success of outsourced bioanalysis for drug development.
Lean teams should evaluate:
- Depth and breadth of bioanalytical expertise
- Regulatory experience and inspection history
- Level of senior scientific involvement
- Flexibility to adapt as programs evolve
- Clarity, transparency, and responsiveness in communication
When selecting a CRO, lean teams should assess experience with:
- Bioanalytical method development and validation
- Managing challenges in bioanalytical method development
- Advanced technologies such as LC-MS for large molecules
Strong communication and scientific leadership are essential.
The most effective partnerships are built on scientific alignment and shared accountability.
10: The Future of Lean Drug Development Depends on Smart Outsourcing
As drug development models evolve, lean and virtual organizations will continue to drive innovation. In this environment, outsourced bioanalysis for drug development is no longer optional—it is foundational.
As lean and virtual models dominate early innovation, outsourced bioanalysis for drug development is no longer optional. Many organizations now outsource bioanalysis for biotech startups to gain speed, flexibility, and regulatory confidence.
Teams that outsource strategically:
- Advance programs more rapidly
- Reduce operational and regulatory risk
- Improve decision-making confidence
- Preserve capital efficiency
ResolveMass Laboratories Inc. supports this future by delivering bioanalytical excellence combined with strategic insight.
Conclusion
Outsourced bioanalysis for drug development enables lean teams to compete with larger organizations by providing access to advanced expertise, regulatory-ready infrastructure, and scalable analytical capacity.
By partnering with ResolveMass Laboratories Inc. and leveraging comprehensive bioanalytical services, drug developers gain clarity, confidence, and control over their development journey.
For teams building smarter, leaner, and faster, outsourced bioanalysis is not just critical—it is transformative.
Frequently Asked Questions:
Risks assessed during outsourcing include quality and regulatory compliance risks, technical capability risks, data integrity and confidentiality risks, timeline and capacity risks, and financial or vendor reliability risks. Proper risk assessment ensures outsourced activities meet scientific, regulatory, and business expectations.
The application of Quality by Design (QbD) has been encouraged by regulatory guidelines (ICH Q8–Q10), increasing product and process complexity, the need for better risk management, and the demand for consistent quality throughout the product lifecycle.
Key challenges of outsourcing include loss of direct control, communication and coordination issues, maintaining consistent quality, effective knowledge transfer, and ensuring regulatory compliance across external partners.
Common outsourcing mistakes include choosing vendors based solely on cost, unclear scope and responsibilities, insufficient oversight, poor communication, and failure to treat outsourcing as a strategic partnership.
The principles of outsourcing include clear definition of scope and objectives, retention of sponsor accountability, selection of qualified and compliant partners, effective communication and governance, and a quality-driven, partnership-based approach.
Reference
- Yan Song, Raj Dhodda, Jun Zhang &Jens Sydor.A High Efficiency, High Quality and Low Cost Internal Regulated Bioanalytical Laboratory to Support Drug Development Needs.https://www.tandfonline.com/doi/abs/10.4155/bio.14.104
- Tom Verhaeghe.Bioanalytical Outsourcing Strategy at Janssen Research and Development.https://www.tandfonline.com/doi/abs/10.4155/bio.14.105
- Stephen Lowes.Outsourcing in Bioanalysis: A CRO Perspective.https://www.tandfonline.com/doi/full/10.4155/bio-2017-4994
- Craig Stovold, Fiona Milligan, Glen Hawthorne, Colin Pattison &Amanda Wilson.Changing Shape: Evolving an Outsourced Bioanalytical Strategy to Support The Changing Needs of Drug Development.https://www.tandfonline.com/doi/full/10.4155/bio-2017-0098
- An Integrated Outsourcing Practice of Nonclinical LC–MS Bioanalysis and Toxicokinetics at Novartis small Molecule Drug Development.https://www.tandfonline.com/doi/abs/10.4155/bio-2021-0072
- Kevin P Bateman, Lucinda Cohen, Bart Emary &Vincenzo Pucci.Standardized Workflows for Increasing Efficiency and Productivity in Discovery Stage Bioanalysis.https://www.tandfonline.com/doi/abs/10.4155/bio.13.162

