Summary
- Two core synthesis strategies—stepwise solid-phase assembly and post-synthetic parallel conjugation—enable flexible design based on sequence length, complexity, and scale.
- Stepwise solid-phase methods provide tight structural control, while parallel conjugation improves scalability and compatibility with longer or modified peptides.
- Strategic linker engineering (cleavable or non-cleavable) controls stability, release timing, biodistribution, and overall therapeutic performance.
- Peptide targeting technologies enhance delivery beyond the liver, enabling precise uptake in muscle, kidney, CNS, and tumor tissues.
- Comprehensive analytics (HRMS, MS/MS, HPLC, ICP-MS, CE) ensure sequence integrity, impurity control, and regulatory-ready quality.
- GMP-aligned scale-up and process optimization support efficient manufacturing, regulatory compliance, and reliable clinical supply.
Advanced Synthesis Strategies in Peptide Oligonucleotide Conjugation Services
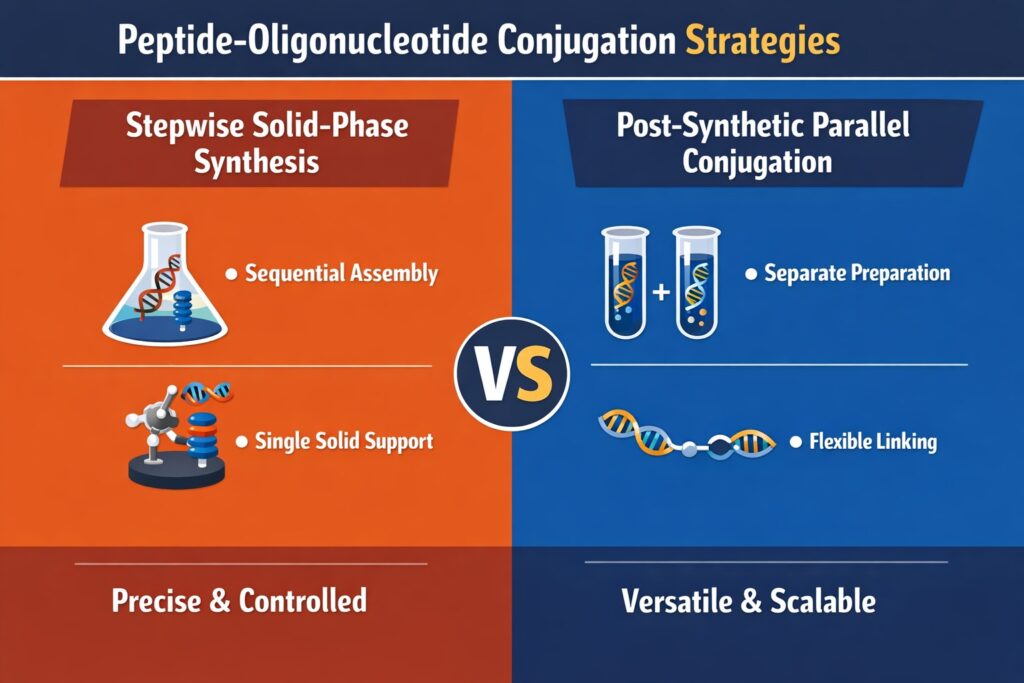
The scientific foundation of Peptide Oligonucleotide Conjugation Services is built upon two main synthetic approaches: stepwise solid-phase synthesis and post-synthetic parallel conjugation. The choice between these strategies depends on several factors, including peptide length, oligonucleotide backbone chemistry, scale of production, and overall project complexity. During early development, both methods are often evaluated to determine which provides the best balance of yield, purity, and structural integrity. Careful assessment at this stage reduces downstream risks and supports smoother scale-up. Selecting the right synthetic pathway plays a major role in long-term manufacturability and regulatory success.
Explore Our Expertise: Learn more about our Peptide & Oligonucleotide Conjugation CRO services
Stepwise Solid-Phase Assembly
Stepwise solid-phase synthesis involves assembling both peptide and oligonucleotide components sequentially on the same solid support. In most cases, the peptide portion is synthesized first using a specialized resin such as polystyrene-co-divinylbenzene. Once the peptide chain is complete, oligonucleotide elongation proceeds using standard phosphoramidite chemistry. This unified approach allows very precise control over molecular architecture and can reduce intermediate purification steps. Because the entire construct is built in a controlled sequence, it offers strong structural definition. However, this method requires highly coordinated protection and deprotection strategies to maintain stability throughout synthesis.
One of the most significant challenges in this method is the incompatibility between peptide and oligonucleotide chemistries. Acidic reagents like trifluoroacetic acid, which are commonly used for peptide deprotection, can damage acid-sensitive oligonucleotide groups such as dimethoxytrityl (DMT) and glycosidic bonds. To address this issue, orthogonal protecting groups and specially designed base-labile linkers are used. These linkers, often containing ester functionalities, can be cleaved under mild ammonia or ethanolamine conditions without harming the conjugate. Continuous refinement of coupling efficiency and deprotection timing further improves yield and product quality. When properly optimized, this approach is highly effective for shorter and structurally defined conjugates.
Post-Synthetic Parallel Conjugation
Post-synthetic parallel conjugation follows a different strategy in which the peptide and oligonucleotide are synthesized and purified separately before being chemically linked. This method provides flexibility, especially when working with long peptides, complex modifications, or even full-length proteins. Because each component is prepared independently, scientists can optimize reaction conditions for each part without cross-interference. This reduces troubleshooting complexity and supports higher scalability. After purification, the two components are combined under carefully controlled reaction conditions to form the final conjugate.
The coupling reactions are typically performed in aqueous or mixed organic-aqueous solvent systems such as acetonitrile or DMSO blends. These solvents help maintain solubility of both the hydrophilic oligonucleotide and the hydrophobic peptide segments. Bioorthogonal reactions such as copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) are widely used due to their efficiency and regioselectivity. However, to avoid concerns about residual copper in clinical materials, copper-free approaches like SPAAC and thiol-maleimide Michael additions are increasingly preferred. These reactions proceed under mild conditions and preserve sensitive functional groups. Proper downstream purification ensures removal of catalysts and unreacted fragments, delivering high-purity conjugates suitable for therapeutic use.
Our Solutions: Discover our specialized POCs Synthesis Services
| Synthesis Strategy | Advantages | Primary Limitations |
| Stepwise Solid-Phase | Precise structural control; internal peptide placement; single purification step. | Protecting group incompatibility; restricted to shorter sequences; potentially lower yields. |
| Post-Synthetic Parallel | Compatible with long peptides/proteins; independent fragment purification; versatile chemistries. | Requires multistep purification; solubility challenges for hydrophobic peptides; potential for side reactions. |
Strategic Linker Selection in Peptide Oligonucleotide Conjugation Services
In Peptide Oligonucleotide Conjugation Services, linker design plays a central role in determining both stability and therapeutic performance. The linker acts as a bridge between the targeting peptide and the oligonucleotide payload, directly influencing release timing and biodistribution. Selecting the correct linker requires understanding the therapeutic mechanism, intracellular environment, and safety profile needed for the target indication. A well-designed linker ensures that the conjugate remains stable in circulation but releases its payload efficiently inside target cells. This balance is essential for maximizing efficacy while minimizing off-target effects.
Cleavable Linker Mechanisms
Cleavable linkers are engineered to respond to specific intracellular triggers such as enzymatic activity, reducing environments, or acidic pH conditions. Disulfide linkers take advantage of elevated intracellular glutathione levels, allowing selective release once inside the cytosol. Stabilized disulfide variants, including gem-dimethyl substitutions, reduce premature cleavage in plasma while maintaining intracellular responsiveness. Enzyme-sensitive linkers such as Valine-Citrulline (Val-Cit) are processed by lysosomal enzymes like Cathepsin B. Many of these systems include para-aminobenzyl (PAB) spacers that enable traceless release of the oligonucleotide, preserving its compatibility with cellular machinery such as the RISC complex. Controlled cleavage mechanisms significantly improve intracellular potency and gene modulation efficiency.
Non-Cleavable Linker Stability
Non-cleavable linkers such as thioethers and triazoles provide exceptional systemic stability. Instead of relying on chemical triggers, these linkers depend on intracellular proteolytic degradation of the peptide portion to activate the therapeutic payload. The oligonucleotide remains attached to a small residual group that does not interfere with hybridization or gene-binding activity. This approach is particularly effective for antisense oligonucleotides (ASOs) and steric-blocking agents. By preventing premature release, non-cleavable linkers reduce systemic exposure to free nucleic acids and help improve safety profiles. Their robustness makes them attractive for long-acting therapeutic designs.
| Linker Category | Representative Examples | Trigger Mechanism | Optimal Application |
| Enzyme-Cleavable | Val-Cit-PAB; Ester; Amide. | Cathepsin B; Lysosomal proteases. | siRNA; payloads requiring “traceless” release. |
| Redox-Cleavable | Disulfide; Gem-dimethyl disulfide. | Cytosolic Glutathione (GSH). | Intracellular gene silencing. |
| Acid-Cleavable | Hydrazone; Acetal; Carbonate. | Low pH (Endosomes/Lysosomes). | Targeted delivery via endocytosis. |
| Non-Cleavable | Thioether; Triazole; PEG. | Proteolytic degradation of peptide. | ASOs; steric blockers; long-acting conjugates. |
Therapeutic Applications of Peptide Oligonucleotide Conjugation Services
The therapeutic scope of Peptide Oligonucleotide Conjugation Services continues to expand as researchers overcome delivery barriers beyond the liver. Historically, many oligonucleotide therapies accumulated primarily in hepatic tissue due to natural receptor-mediated uptake. However, peptide conjugation now enables selective delivery to extrahepatic tissues such as kidney, skeletal muscle, and even the central nervous system. This tissue-directed approach improves therapeutic concentration at disease sites while lowering systemic exposure. As a result, treatment precision and overall therapeutic index are significantly enhanced.
Accelerate Discovery: Custom Synthesis services for Drug Discovery
Extrahepatic Tissue Targeting
Peptide-mediated targeting enables improved uptake in tissues that were previously difficult to reach. For example, kidney-targeting peptides utilize proximal tubule reabsorption pathways to increase local ASO concentration substantially. This improved localization enhances inhibition of targets such as HIF-1α while limiting off-target accumulation. In Duchenne muscular dystrophy (DMD), cell-penetrating peptide (CPP) conjugates linked to modified oligonucleotide backbones promote exon skipping and increase dystrophin production in muscle cells. These developments highlight the transformative role of peptide-guided nucleic acid delivery. Expanding tissue access opens opportunities for treating neuromuscular, renal, and neurodegenerative diseases.
Cell-Penetrating and Homing Peptides
Peptides used in conjugation provide multiple functional advantages for cellular uptake and intracellular trafficking. Arginine- and lysine-rich CPPs enhance membrane translocation through electrostatic interactions and macropinocytosis pathways. Tumor-targeting peptides such as RGD selectively bind integrins that are overexpressed on cancer cells, enabling receptor-mediated internalization. Endosomal escape peptides like GALA and INF7 help overcome intracellular degradation barriers by disrupting membranes under acidic conditions. This improves cytosolic release and increases effective gene modulation. Continuous peptide engineering is refining both targeting precision and safety outcomes.
| Peptide Class | Mechanism of Action | Exemplary Application |
| Cell-Penetrating (CPP) | Membrane translocation; macropinocytosis. | siRNA/ASO delivery; DMD exon skipping. |
| Tissue-Homing | Receptor-mediated endocytosis. | Renal tubular targeting; Tumor-specific imaging. |
| Endosomal Escape | pH-sensitive membrane perturbation. | Increasing cytosolic bioavailability of siRNAs. |
| Nuclear Localization (NLS) | Importin-mediated nuclear transport. | Antisense inhibition of nuclear-retained RNA. |
| Organelle-Targeting | Signal sequence recognition. | Mitochondrial gene regulation; lysosomal research. |
Analytical Characterization within Peptide Oligonucleotide Conjugation Services
Comprehensive analytical characterization is essential for confirming sequence identity, structural integrity, and purity of peptide-oligonucleotide conjugates. Due to the hybrid nature of these molecules, analytical workflows must evaluate both peptide and nucleic acid components simultaneously. High-resolution mass spectrometry (HRMS) remains the gold standard for molecular confirmation. Combining orthogonal analytical techniques increases confidence in structural assignments. Robust analytical control supports regulatory submissions and long-term product consistency.
Quality Assurance: View our detailed Peptide Characterization Service
Sequence Confirmation and Impurity Profiling
Techniques such as ESI-MS/MS and MALDI-TOF MS allow detection of subtle backbone changes, including phosphorothioate (PS) to phosphodiester (PO) substitutions. Ultra-high-resolution systems can distinguish closely related n-1 and n+1 sequence variants with high precision. Controlled capping strategies during synthesis help reduce n-1 impurities to below 1%, which is critical for therapeutic applications. Accurate impurity profiling ensures consistent performance and minimizes safety concerns. This level of analytical rigor is necessary for clinical-grade materials.
Advanced Chromatographic and Elemental Techniques
High-performance liquid chromatography (HPLC) using ion-pair reverse-phase (IP-RP) or anion-exchange (AEX) modes remains central to purity assessment. Capillary LC-ICPMS enables selective detection of phosphorus (^31P), allowing accurate quantification of oligonucleotide components in conjugates. This method is highly sensitive and sequence-independent. Melting temperature (Tm) analysis evaluates duplex stability for siRNA constructs and confirms functional integrity. CE-SDS supports size-based purity assessment and Drug-to-Antibody Ratio (DAR) determination for larger bioconjugates.
Mass Spectrometry Solutions: Advanced Mass Spec Analysis for research and development
GMP Scale-Up for Peptide Oligonucleotide Conjugation Services
Transitioning from laboratory research to commercial-scale production requires a strong Chemistry, Manufacturing, and Controls (CMC) framework. Compliance with ICH Q6A and Q11 guidelines ensures structured development and documentation. Automated synthesis platforms enhance reproducibility and minimize batch variability. During scale-up, purification capacity, stereochemical control, and impurity management must be carefully monitored. Strategic planning at this stage reduces regulatory risk and supports uninterrupted clinical supply.
Process Intensification and Yield Optimization
Modern manufacturing platforms incorporate automation and continuous chromatography to improve efficiency. Twin-column chromatography systems can reduce purification costs by 30% to 50%, making large-scale production more economical. Integration of ion-pair reverse-phase and ion-exchange purification steps streamlines workflows and shortens processing times. Reduced manual handling also lowers contamination risk. These process improvements contribute to sustainable and scalable production.
Regulatory Framework and Quality Assurance
Oligonucleotide therapeutics are regulated as chemical entities but share biological complexity, requiring rigorous GMP compliance. Facilities must operate under validated systems and follow ALCOA principles to ensure data integrity. Stability studies conducted under stress and long-term conditions confirm product robustness. Regulatory agencies such as FDA and EMA expect detailed, phase-appropriate documentation. Strong quality systems ensure readiness for inspection and global approval pathways.
Overcoming Endosomal Barriers in Conjugate Design
A major limitation in nucleic acid therapy is that only a small fraction of internalized material reaches the cytosol. Improving endosomal escape remains a central focus in Peptide Oligonucleotide Conjugation Services. Without effective escape, therapeutic potency is significantly reduced. Researchers are developing pH-responsive peptides and small-molecule enhancers to address this challenge. Enhancing cytosolic release directly correlates with improved gene modulation efficiency.
Mechanistic Insights into Escape Peptides
Peptides such as INF7 and GALA adopt alpha-helical structures under acidic endosomal conditions. This conformational change enables controlled membrane disruption and release of the conjugate into the cytoplasm. Histidine-rich peptides utilize the proton sponge effect, causing osmotic swelling and rupture of endosomes. Structural fine-tuning improves selectivity while reducing toxicity. Balanced membrane interaction is essential for maintaining safety.
Future Trends and Computational Modeling
Computational modeling and machine learning tools are increasingly used to predict uptake efficiency and intracellular release behavior. Bayesian approaches support rational design by identifying structural features linked to performance. Advanced imaging technologies now allow visualization of individual escape events in real time. Emerging chemistries such as SuFEx click reactions offer new stability advantages. These innovations are shaping the next generation of precision-designed conjugates.
Conclusion
Peptide Oligonucleotide Conjugation Services represent a transformative platform in therapeutic development by addressing both biological delivery barriers and chemical manufacturing complexities. Through advanced synthesis strategies, intelligent linker engineering, comprehensive analytical validation, and GMP-compliant scale-up, these services enable production of highly pure and targeted nucleic acid therapeutics. From early discovery to regulatory approval, structured development ensures clinical readiness and long-term product reliability. As delivery technologies and computational tools continue to advance, these services will further expand treatment possibilities across complex genetic, metabolic, and degenerative diseases.
For professional inquiries and to begin your next therapeutic development project, please contact our expert team:
Most Asked FAQs on Peptide-Oligonucleotide Conjugation
Post-synthetic conjugation allows the peptide and oligonucleotide to be produced and purified separately before they are joined together. This gives researchers better control over quality and makes it easier to work with long or structurally complex peptides and proteins. It also avoids exposing sensitive biomolecules to harsh chemical conditions used during in-line synthesis. As a result, this approach is highly flexible and well suited for advanced therapeutic designs.
In stepwise synthesis, scientists use specially designed protecting groups and base-cleavable linkers to shield the oligonucleotide from acidic conditions required in peptide chemistry. These protective strategies prevent unwanted degradation during assembly. In parallel synthesis, both fragments are prepared independently and then connected using gentle bioorthogonal reactions. This ensures that both the peptide and the oligonucleotide remain structurally intact.
“n-1” impurities are shortened sequences that are missing one nucleotide. Even though they may appear in small amounts, they can potentially reduce efficacy or cause unintended biological effects. Therefore, strict quality control measures are applied to minimize their presence. Advanced purification methods and high-resolution mass spectrometry are used to keep these impurities within acceptable regulatory limits.
Cleavable linkers are preferred when the therapeutic payload must be fully released inside the cell to function properly. For example, siRNA therapies require complete separation from the targeting peptide to enter the RISC complex and silence genes effectively. Non-cleavable linkers are more suitable for antisense oligonucleotides (ASOs), where a small residual fragment does not interfere with binding. The choice depends on the mechanism of action and therapeutic goal.
The peptide component can be engineered to guide the conjugate toward specific tissues or cell types. Targeting peptides may bind to receptors such as integrins or glucose transporters that are highly expressed in certain organs. This allows delivery beyond the liver, including tissues like brain, muscle, or kidneys. Proper peptide design significantly improves tissue specificity and treatment precision.
Ultra-high-resolution mass spectrometry methods such as ESI-MS/MS and MALDI-TOF MS are commonly used for structural confirmation. These techniques help identify modified nucleotides, backbone changes, and exact conjugation sites. They also detect small sequence variations or positional isomers. Accurate analytical validation ensures product integrity and regulatory compliance.
For peptide–oligonucleotide conjugates, the molar ratio between peptide and oligonucleotide is tightly controlled through site-specific chemistry. Analytical methods such as HPLC, UPLC, and UV-Vis spectroscopy are used to confirm uniformity. These techniques ensure batch-to-batch consistency and reproducibility. Maintaining a defined ratio is essential for predictable therapeutic performance.
Certain backbone modifications, such as phosphorothioate linkages, introduce chiral centers into the molecule. Standard synthesis may produce mixtures of stereoisomers, which can show different biological properties. To improve consistency and simplify regulatory evaluation, some manufacturers now offer stereopure synthesis. This approach enhances uniformity and supports clearer pharmacological characterization.
Reference
- Klabenkova, K., Fokina, A., & Stetsenko, D. (2021). Chemistry of peptide-oligonucleotide conjugates: A review. Molecules, 26(17), 5420. https://doi.org/10.3390/molecules26175420
- Alas, M., Saghaeidehkordi, A., & Kaur, K. (2020). Peptide-drug conjugates with different linkers for cancer therapy. Journal of Medicinal Chemistry, 64(1), 216–232. https://doi.org/10.1021/acs.jmedchem.0c01530
- Malinowska, A. L., Huynh, H. L., & Bose, S. (2024). Peptide–oligonucleotide conjugation: Chemistry and therapeutic applications. Current Issues in Molecular Biology, 46(10), 11031–11047. https://doi.org/10.3390/cimb46100655