
Introduction:
Peptide Sameness Study Services in Canada are essential for pharmaceutical companies seeking regulatory approval for generic peptide products. Demonstrating “sameness” requires comprehensive analytical characterization proving that the generic peptide is highly similar to the reference listed drug (RLD) in structure, purity, impurities, and critical quality attributes.
At ResolveMass Laboratories Inc., we specialize in advanced LC-MS/MS and high-resolution mass spectrometry–based characterization for peptide therapeutics. Our expertise in peptide characterization in drug development ensures regulatory-ready data packages aligned with Health Canada, US FDA, and international expectations.
For detailed regulatory alignment, refer to:
- Peptide characterization for IND and NDA submissions
- FDA requirements for peptide characterization
- Characterization of peptides for FDA submissions
Summary:
- Peptide Sameness Study Services in Canada are critical for ANDA and generic peptide drug approvals.
- Regulatory agencies require extensive structural, physicochemical, and impurity profiling beyond simple bioequivalence.
- Advanced LC-MS/MS, HRMS, peptide mapping, and orthogonal characterization techniques are essential.
- ResolveMass Laboratories Inc. provides regulatory-aligned peptide sameness studies tailored to Health Canada, FDA, and global submissions.
- A scientifically rigorous sameness strategy reduces regulatory risk, deficiency letters, and approval delays.
For ANDA-specific guidance, explore:
1: What Are Peptide Sameness Study Services in Canada?
Peptide Sameness Study Services in Canada are analytical programs designed to demonstrate that a generic peptide drug is structurally and functionally equivalent to its reference product. These studies are mandatory for regulatory submissions such as ANDA or abbreviated pathways.
Unlike small molecules, peptides:
- Have complex higher-order structures
- May contain sequence variants and truncations
- Are prone to oxidation, deamidation, and aggregation
- Often require detailed impurity profiling
A complete sameness study typically includes:
| Study Component | Purpose | Techniques Used |
|---|---|---|
| Primary structure confirmation | Verify amino acid sequence | LC-MS/MS, HRMS |
| Peptide mapping | Confirm sequence coverage | Enzymatic digestion + LC-MS |
| Impurity profiling | Detect process-related impurities | UPLC, LC-MS |
| Related substances | Quantify degradants | Stability-indicating HPLC |
| Aggregation analysis | Evaluate higher-order issues | SEC, MS |
Understanding manufacturing impact is critical. Learn more about:
A complete sameness study includes mapping, impurity profiling, degradation assessment, and purity testing:
- Peptide mapping vs peptide sequencing
- Peptide mapping for PTM analysis
- Peptide degradation product characterization
- Peptide purity by HPLC and why it matters
Peptide Sameness Study Services in Canada must combine multiple orthogonal methods to satisfy regulatory scrutiny.
2: Why Are Peptide Sameness Study Services in Canada Critical for Regulatory Approval?
They are critical because regulatory agencies require detailed structural and impurity comparison for peptides before granting approval.
Regulators recognize that peptides are more complex than traditional small molecules. Therefore:
- Bioequivalence alone is insufficient.
- Minor sequence variations can impact safety and efficacy.
- Impurity profiles must closely match the RLD.
Health Canada and global authorities expect:
- Comprehensive analytical characterization
- Comparative impurity qualification
- Forced degradation studies
- Stability data
A poorly designed sameness study may result in:
- Deficiency letters
- Delays in approval
- Additional costly studies
For deeper insight into impurity evaluation:
If unexpected species arise:
Selecting the right CRO is critical:
- How to choose the right peptide synthesis service partner
- Top 5 things to look for in a peptide testing laboratory
- Peptide testing services for pharmaceutical R&D
ResolveMass Laboratories Inc. designs peptide sameness strategies aligned with global regulatory expectations to minimize submission risk.
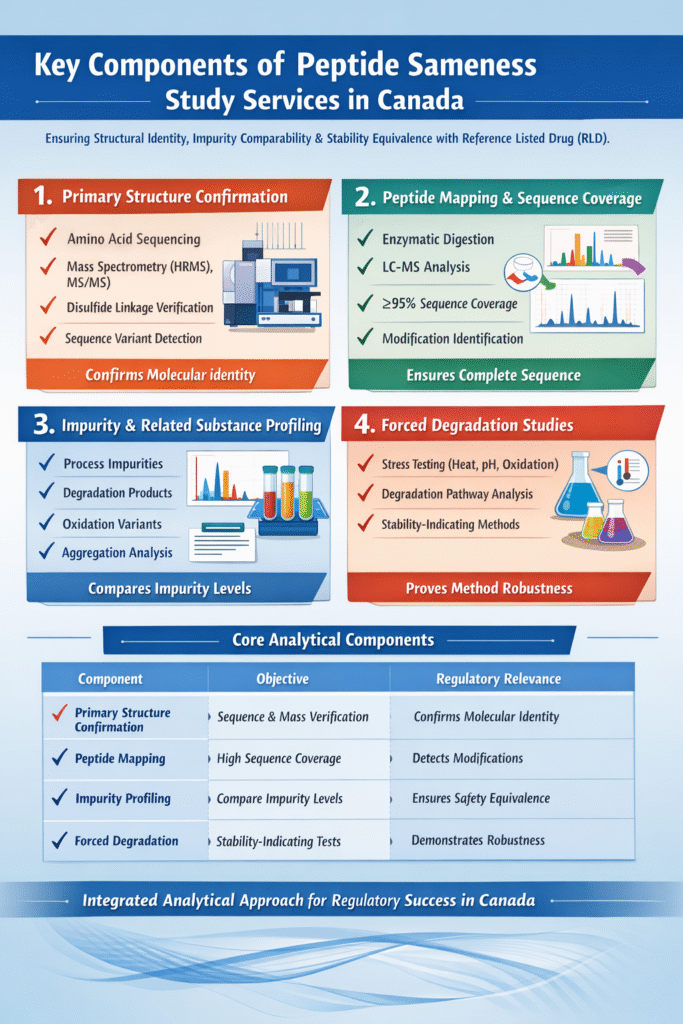
3: Key Components of Peptide Sameness Study Services in Canada
Peptide Sameness Study Services in Canada require a multi-layered analytical approach to demonstrate structural identity, impurity comparability, and stability equivalence with the reference listed drug (RLD). Below are the four core pillars regulators expect in a robust sameness package.
1. Primary Structure Confirmation
Primary structure confirmation verifies the exact amino acid sequence and molecular weight of the peptide to ensure structural identity with the reference product.
Primary structure confirmation relies on advanced mass spectrometry techniques delivered by peptide mass spectrometry experts.
This is the foundational requirement in Peptide Sameness Study Services in Canada, as even a single amino acid variation can alter biological activity or immunogenicity.
We utilize:
- High-resolution mass spectrometry (HRMS)
- Tandem MS (MS/MS fragmentation analysis)
- Accurate intact mass determination
- Isotopic pattern verification
This ensures confirmation of:
- Correct amino acid sequence
- Absence of truncations or extensions
- Verification of disulfide linkages (if applicable)
- Detection of sequence variants
Case study examples:
- Ganirelix generic peptide characterization project
- Lanreotide generic peptide characterization project
Regulatory Importance: Authorities expect clear spectral overlays and mass accuracy within acceptable ppm limits, supported by documented interpretation.
2. Peptide Mapping and Sequence Coverage
Peptide mapping confirms sequence identity through enzymatic digestion followed by LC-MS analysis, providing detailed sequence-level verification.
This is one of the most scrutinized components of Peptide Sameness Study Services in Canada because it demonstrates sequence coverage and identifies subtle modifications.
Typical Workflow:
- Enzymatic digestion (e.g., trypsin or alternative proteases)
- Chromatographic separation of fragments
- MS-based fragment identification
- Comparative mapping against reference standard
Outcomes Achieved:
- ≥95–100% sequence coverage
- Detection of sequence variants
- Identification of post-translational or chemical modifications
- Confirmation of disulfide bond patterns (if applicable)
Explore:
Regulatory Expectation: Comparative chromatograms, fragment maps, and tabulated sequence coverage data must be presented clearly in submission-ready format.
3. Impurity and Related Substance Profiling
Impurity profiling quantitatively compares related substances between the test and reference product to ensure comparable safety profiles.
Regulators require a side-by-side impurity assessment as part of Peptide Sameness Study Services in Canada because peptides are prone to degradation and process-related impurities.
We evaluate:
- Process-related impurities
- Degradation products
- Oxidation variants
- Deamidated species
- Truncated peptides
- Aggregates (where applicable)
Analytical Techniques Used:
- UPLC for high-resolution separation
- LC-MS/MS for structural elucidation
- High-sensitivity detection at trace levels
- Orthogonal confirmation where required
Impurity comparability ensures safety equivalence.
- Impurity profiling in peptides – why it matters in drug development
- Peptide purity testing in the United States
Key Objective: Demonstrate that impurity levels fall within acceptable thresholds and are comparable to the RLD or scientifically justified.
4. Forced Degradation Studies
Forced degradation studies establish that the analytical method is stability-indicating and characterize degradation pathways under stress conditions.
These studies are essential within Peptide Sameness Study Services in Canada to prove that analytical methods can detect degradation differences reliably.
Stress Conditions Commonly Applied:
- Acid and base hydrolysis
- Oxidative stress
- Thermal stress
- Photolytic exposure
These Studies Confirm:
- Method specificity
- Stability-indicating capability
- Degradation pathway comparability
- Identification of major degradants
Degradation pathway understanding is essential for stability-indicating methods.
Regulatory Insight: Authorities often review degradation profiles carefully to ensure no unexpected degradants appear in the generic product.
4: How ResolveMass Laboratories Inc. Ensures Scientific Rigor
ResolveMass applies advanced analytical platforms, regulatory-aligned SOPs, and expert interpretation to deliver submission-ready data.
Our capabilities include:
- High-resolution LC-MS/MS platforms
- Orthogonal analytical methods
- Method validation per ICH guidance
- Regulatory documentation support
What Makes Our Approach Different?
- Experienced scientists in peptide bioanalysis
- Deep understanding of regulatory expectations
- Structured gap assessment before study initiation
- Clear data interpretation reports
We do not simply generate data—we provide regulatory-contextualized interpretation.
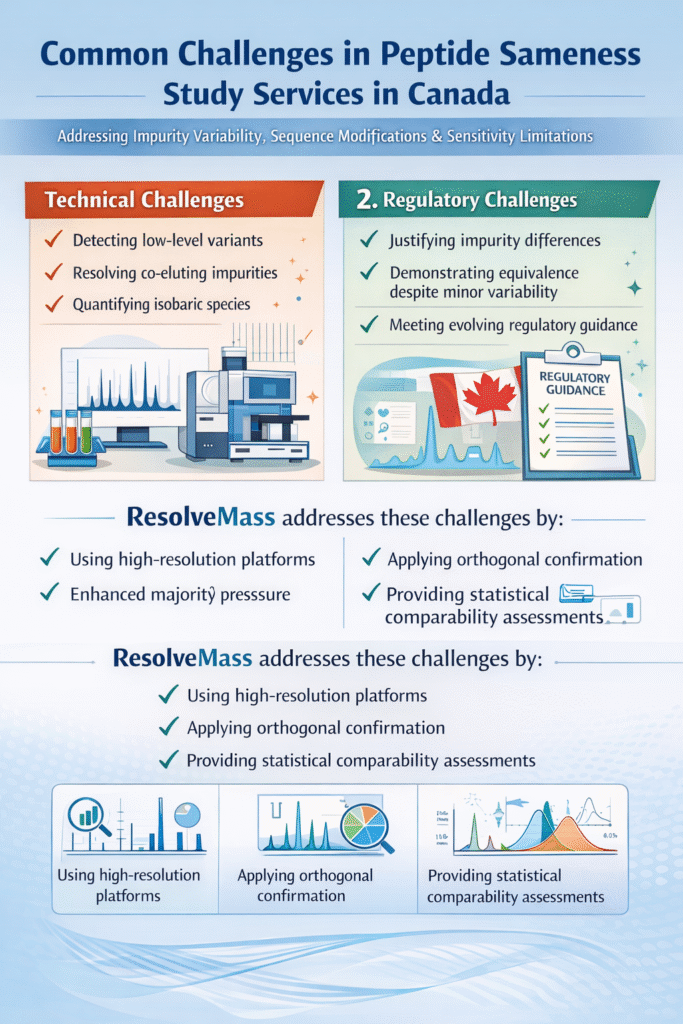
5: Common Challenges in Peptide Sameness Study Services in Canada
The most common challenges include impurity variability, sequence modifications, and analytical sensitivity limitations.
Technical Challenges
- Detecting low-level variants
- Resolving co-eluting impurities
- Quantifying isobaric species
Regulatory Challenges
- Justifying impurity differences
- Demonstrating equivalence despite minor variability
- Meeting evolving regulatory guidance
ResolveMass addresses these challenges by:
- Using high-resolution platforms
- Applying orthogonal confirmation
- Providing statistical comparability assessments
6: Regulatory Expectations for Peptide Sameness Study Services in Canada
Regulators expect comprehensive analytical comparability rather than minimal equivalence.
Key regulatory focus areas:
- Structural identity
- Impurity comparability
- Stability comparability
- Batch-to-batch consistency
Authorities may request:
- Side-by-side chromatograms
- Overlay spectral data
- Quantitative impurity comparison tables
A strong regulatory submission includes:
- Clear methodology
- Validated analytical methods
- Well-organized technical reports
7: Why Choose ResolveMass for Peptide Sameness Study Services in Canada?
ResolveMass Laboratories Inc. provides specialized peptide-focused analytical services with regulatory alignment and technical depth.
Our Strengths
- Advanced LC-MS/MS expertise
- Canadian laboratory infrastructure
- Regulatory documentation support
- Customized study design
Our Commitment to Quality
- Strict data integrity compliance
- Validated methods
- QA-reviewed reports
- Transparent communication
We understand that peptide approvals demand precision, defensible data, and strategic scientific planning.
Conclusion:
Peptide Sameness Study Services in Canada are a critical component of successful generic peptide drug development. Regulatory authorities require comprehensive structural confirmation, impurity profiling, and stability comparison to demonstrate true equivalence to the reference product.
ResolveMass Laboratories Inc. delivers scientifically rigorous, regulatory-ready peptide sameness studies designed to reduce risk, accelerate approvals, and ensure submission success. If you are planning an ANDA or generic peptide submission, investing in high-quality analytical characterization is not optional—it is essential.
Frequently Asked Questions:
No, bioequivalence alone is not sufficient for peptide approval.
Unlike small-molecule drugs, peptides are structurally complex and highly sensitive to minor variations. Regulatory authorities such as Health Canada and the U.S. Food and Drug Administration require comprehensive analytical characterization in addition to pharmacokinetic bioequivalence studies.
For peptide generics, regulators expect demonstration of:
-Exact amino acid sequence confirmation
-High sequence coverage via peptide mapping
-Comparable impurity and related substance profiles
-Stability-indicating method validation
-Forced degradation comparability
Even small differences in sequence variants, oxidation, deamidation, or impurity levels can affect safety, efficacy, or immunogenicity. Therefore, a robust peptide sameness study is mandatory to demonstrate true structural and physicochemical equivalence to the reference listed drug (RLD).
Peptide Sameness Study Services in Canada are analytical programs designed to demonstrate that a generic peptide drug is structurally and functionally equivalent to the reference listed drug (RLD). These studies involve advanced LC-MS/MS, HRMS, peptide mapping, impurity profiling, and stability-indicating assessments to meet regulatory requirements.
Peptides are structurally complex molecules. Even a single amino acid change, oxidation event, or impurity difference can impact safety, efficacy, or immunogenicity. Therefore, regulators require comprehensive structural and impurity analysis in addition to bioequivalence studies.
Common techniques include:
-High-resolution mass spectrometry (HRMS)
-LC-MS/MS
-Peptide mapping
-UPLC/HPLC impurity profiling
-Stability-indicating methods
-Forced degradation studies
-Orthogonal confirmation techniques
Reference
- Recommendation for Clarifying FDA Policy in Evaluating “Sameness” of Higher Order Structure for Generic Peptide Therapeutics.https://link.springer.com/article/10.1208/s12248-024-00994-8
- Building parity between brand and generic peptide products: Regulatory and scientific considerations for quality of synthetic peptides.https://www.sciencedirect.com/science/article/abs/pii/S0378517316311930
- Canadian Proteomics: A Journey across the Country Highlights Discovery and Innovation.https://pubs.acs.org/doi/full/10.1021/acs.jproteome.4c00960
- Peptide Therapeutics and the Pharmaceutical Industry: Barriers Encountered Translating from the Laboratory to Patients.https://www.ingentaconnect.com/content/ben/cmc/2016/00000023/00000037/art00004

