Introduction:
In modern drug development, PK TK bioanalysis is one of the most scrutinized scientific packages reviewed by regulatory agencies. Without reliable exposure and safety data, a promising molecule cannot enter human trials. At ResolveMass Laboratories Inc., we routinely see projects delayed not because the drug failed — but because the bioanalytical data lacked regulatory readiness.
To understand the importance of bioanalysis in development, sponsors should first review why bioanalysis matters in drug development and how it supports drug development programs including IND-enabling bioanalytical studies.
Regulators do not only ask: Does the drug work?
They ask: Can we trust the data proving it works and is safe?
This article explains exactly what authorities expect from CRO-generated bioanalytical datasets and how sponsors can prepare submission-ready studies.
Summary:
- PK TK bioanalysis proves drug exposure, safety margin, and dose selection before human trials.
- Regulators expect validated methods, traceable data integrity, and scientifically justified acceptance criteria.
- CRO data must demonstrate reproducibility, robustness, and transparency — not just passing results.
- Common rejection reasons include poor method validation, stability gaps, and undocumented deviations.
- Early collaboration between sponsors and CRO scientists prevents costly IND delays.
- High-quality PK/TK datasets directly determine whether a drug proceeds to clinical trials.
1: What Is PK TK Bioanalysis and Why Regulators Care
PK TK bioanalysis measures drug concentration in biological matrices to establish exposure-response and safety margins. This work typically includes PK/PD bioanalysis , toxicokinetic bioanalysis, and clinical bioanalytical services.
Pharmacokinetics determines how the body processes the drug, while toxicokinetics confirms exposure levels in safety studies. Together they form the bridge between preclinical safety and first-in-human dosing.
Key Regulatory Questions Answered by PK TK Bioanalysis
- Did the test species receive adequate exposure?
- Is NOAEL exposure higher than clinical starting dose?
- Are metabolites covered in toxicology studies?
- Are accumulation and clearance predictable?
- Are bioanalytical methods reliable?
Without these answers, regulators cannot approve human testing.
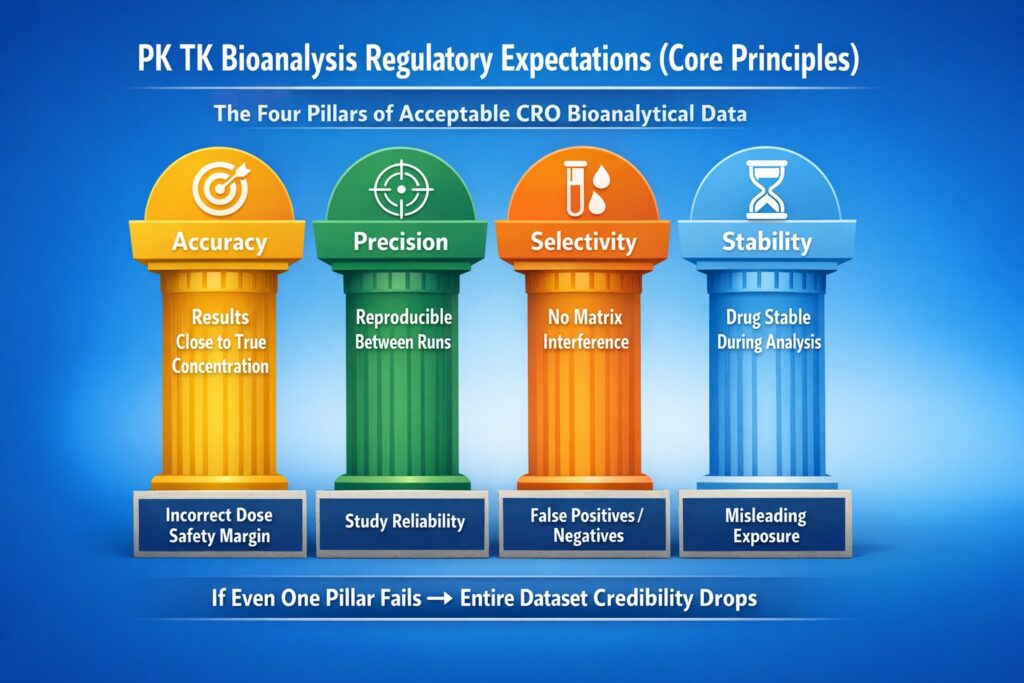
2: PK TK Bioanalysis Regulatory Expectations (Core Principles)
Regulators expect scientifically justified, validated, traceable, and reproducible data — not just compliant reports. This is why companies must understand the difference between discovery vs regulated bioanalysis and work with regulated bioanalytical CRO partners.
The Four Pillars of Acceptable CRO Bioanalytical Data
| Pillar | What It Means | Regulatory Concern |
|---|---|---|
| Accuracy | Results close to true concentration | Incorrect dose safety margin |
| Precision | Reproducible between runs | Study reliability |
| Selectivity | No matrix interference | False positives/negatives |
| Stability | Drug stable during analysis | Misleading exposure |
If even one pillar fails → entire dataset credibility drops.
3: PK TK Bioanalysis Method Validation Expectations
Validation must demonstrate method performance across real biological variability, not ideal laboratory conditions. Sponsors should design studies using bioanalytical method validation, bioanalytical method development, and understand challenges in method development.
Matrix effects and stability testing are especially common regulatory deficiencies.
Mandatory Validation Parameters
- Calibration curve performance
- Accuracy & precision (intra/inter-day)
- Matrix effects
- Dilution integrity
- Carryover
- Selectivity
- Recovery
- Stability (bench-top, freeze-thaw, long-term)
Typical Acceptance Criteria
| Parameter | Acceptance |
|---|---|
| Accuracy | ±15% (±20% at LLOQ) |
| Precision | ≤15% CV (≤20% at LLOQ) |
| Calibration points | ≥75% within limits |
| Stability | Within ±15% |
4: PK TK Bioanalysis in GLP Toxicology Studies
TK analysis confirms that toxicology animals were adequately exposed to the drug.
A toxicity study without exposure confirmation is scientifically meaningless. Regulators specifically check whether observed toxicity correlates with exposure levels.
Toxicokinetic analysis confirms that toxicology animals were adequately exposed to the drug. For complex therapeutics such as cell & gene therapy bioanalysis , antibody drug conjugates , or biosimilars , exposure confirmation becomes even more critical.
What Reviewers Examine Closely
- Satellite vs main group sampling design
- Sparse vs serial sampling justification
- Accumulation over repeat dosing
- Gender exposure differences
- Nonlinear kinetics
5: Common CRO Mistakes That Cause Regulatory Rejection
Most bioanalytical failures come from documentation gaps rather than analytical performance.
Most failures come from documentation gaps rather than analytical performance. Proper bioanalytical CRO project management and outsourcing strategy prevents repeat toxicology studies.
Frequent Issues
- Missing reintegration audit trail
- Unjustified batch acceptance
- Stability not matching study duration
- Incomplete metabolite monitoring
- Calibration outside study range
- Manual data correction without explanation
Example Consequence
A toxicology study costing $500k may become unusable — requiring repetition — simply because incurred sample reproducibility (ISR) failed.
6: PK TK Bioanalysis Data Integrity Requirements
Regulators expect traceable, tamper-proof datasets with audit trails. Emerging tools such as AI in bioanalysis and virtual bioanalytical strategy are increasingly used to improve data review consistency and high throughput bioanalysis .
Data Integrity Elements
- Electronic audit trail
- Chromatogram traceability
- Documented reintegration rules
- Version-controlled SOPs
- Analyst training records
If regulators suspect selective reporting, the dataset loses credibility regardless of results.
7: PK TK Bioanalysis and Metabolite Coverage
Major human metabolites must be present in toxicology exposure data. Advanced LC-MS/MS xenobiotic bioanalysis and high-sensitivity LC-MS/MS bioanalytical services are commonly required to detect low-level metabolites.
If a metabolite exceeds 10% of parent exposure in humans but is absent in animals, additional safety studies may be required.
Regulatory Focus
- Unique human metabolites
- Reactive metabolites
- Active metabolites
- Exposure multiples
8: How PK TK Bioanalysis Determines First-in-Human Dose
Starting clinical dose is calculated using exposure margins from TK NOAEL. This requires reliable bioanalytical quantification and biomarker bioanalytical services including advanced biomarker strategies.
Simplified Dose Logic
- Identify NOAEL exposure in animals
Determine the highest dose in toxicology studies where no adverse effects occur and confirm the actual exposure using TK data. - Convert to Human Equivalent Dose (HED)
Apply body surface area or allometric scaling to translate animal exposure into a safe starting human exposure estimate. - Apply Safety Factors
Introduce regulatory safety margins (commonly 10× or greater) to protect first-in-human participants. - Confirm Analytical Reliability
Verify the bioanalytical assay is validated, stable, and reproducible — regulators rely on assay credibility before accepting the calculated dose.
If the bioanalysis is unreliable → the dose calculation becomes scientifically invalid and regulators may reject the study.

9: PK TK Bioanalysis Reporting Expectations
Reports must explain science — not just present numbers. IND and NDA submission support and advanced bioanalytical strategies for complex drug modalities are critical for regulatory acceptance.
A Regulatory-Ready Report Includes
- Study rationale
- Method suitability discussion
- Deviations impact assessment
- Scientific justification for exclusions
- Exposure interpretation
A data dump is not a scientific report.
10: Sponsor Checklist Before Submitting CRO Data
Sponsors should review bioanalytical data like regulators do — critically.
Pre-Submission Checklist
- Are validation conditions identical to study?
- Are acceptance failures scientifically justified?
- Does stability cover storage duration?
- Are chromatograms reviewable?
- Does exposure support toxicology conclusions?
Helpful planning resources:
- https://resolvemass.ca/bioanalytical-services/
- https://resolvemass.ca/bioanalytical-laboratory-services/
- https://resolvemass.ca/resolvemass-bioanalytical-services-overview/
Startup-specific guidance:
- https://resolvemass.ca/affordable-bioanalytical-services-for-biotech-startups/
- https://resolvemass.ca/outsource-bioanalysis-for-biotech-startups/
- https://resolvemass.ca/bioanalytical-cro-for-drug-discovery/
Cost planning:
- https://resolvemass.ca/bioanalytical-testing-services-cost/
- https://resolvemass.ca/cost-effective-bioanalytical-services/
11: Future of PK TK Bioanalysis
Agencies increasingly evaluate scientific soundness rather than checklist compliance.
Emerging expectations:
- Cross-species comparability
- Model-informed drug development support
- Biomarker-linked exposure
- Advanced LC-MS specificity
Emerging therapies like oligonucleotide LC-MS bioanalysis () and complex modality programs increasingly require specialized regulated CRO support.
High-quality interpretation is becoming as important as data generation.
Conclusion:
Reliable PK TK bioanalysis is the foundation of regulatory confidence in preclinical drug development. Authorities do not approve drugs based on promising biology alone — they approve scientifically defensible exposure data. A CRO must demonstrate validated methods, transparent documentation, and scientifically interpretable results. When PK TK bioanalysis is executed with regulatory expectations in mind, IND submissions proceed smoothly; when it is treated as routine testing, delays and repeat studies are inevitable.
Frequently Asked Questions:
PK TK bioanalysis ensures that drug exposure, safety margins, and pharmacokinetics in animals are accurately measured. Regulators use this data to confirm that starting doses for humans are safe. Without reliable bioanalytical data, IND submissions may be delayed or rejected. It forms the scientific bridge between preclinical safety and first-in-human trials. Accurate PK/TK data protects both participants and sponsors from regulatory setbacks.
Most rejections are caused by documentation gaps rather than analytical errors. Common issues include missing audit trails, unjustified batch acceptance, incomplete metabolite monitoring, calibration outside study range, and stability data not matching study duration. Even high-quality assays can be rejected if the data is not traceable. These mistakes can result in costly repeat studies and delayed submissions.
CROs prevent rejection by following validated bioanalytical methods and maintaining full documentation. Proper project management ensures all deviations and data corrections are justified. Stability testing, incurred sample reproducibility (ISR), and method verification reduce risks. Early collaboration with sponsors ensures study design meets regulatory expectations. Transparency in reporting strengthens data credibility.
Regulators expect bioanalytical data to be:
-Scientifically justified
-Validated for accuracy, precision, selectivity, and stability
-Traceable and reproducible
-Supported by clear documentation of deviations and method performance
Even highly accurate bioanalytical assays can be rejected if data traceability or explanations for deviations are missing. Documentation shows how results were generated and supports reproducibility. Regulators rely on this transparency to trust study conclusions. Proper records prevent costly repeat studies and help maintain submission timelines. Documentation validates both the process and the data.
Reference
- When Is It Appropriate to Outsource Bioanalysis Work to a CRO?https://www.ppd.com/wp-content/uploads/2022/06/Pharm-Tech-2022.06-BioA-outsourcing-1.pdf
- Stephen Lowes. Outsourcing in Bioanalysis: A CRO Perspective.https://www.tandfonline.com/doi/full/10.4155/bio-2017-4994
- John S. Kendrick &Colin Webber. One small step in time, one giant leap for DMPK kind – a CRO perspective of the evolving core discipline of drug development.https://www.tandfonline.com/doi/abs/10.1080/00498254.2022.2124389
- Alexandra Georgiou, Kelly Dong, Stephen Hughes &Matthew Barfield.An Interlaboratory Transfer of A Multi-Analyte Assay Between Continents.https://www.tandfonline.com/doi/abs/10.4155/bio.15.27

