
INTRODUCTION
At ResolveMass Laboratories Inc., we understand that a robust PLGA Degradation Profile is fundamental to the success of any PLGA‑based drug delivery system. The term “PLGA Degradation Profile” refers to how the PLGA polymer degrades over time in a given environment, which in turn dictates drug release kinetics, stability of the drug–polymer matrix, and overall safety of the formulation. In this article, we describe how the PLGA Degradation Profile influences drug release, safety, and stability — offering actionable insights for formulation scientists, regulatory reviewers, and pharmaceutical developers alike.
For a deeper dive into PLGA stability challenges, you may also read our detailed guide on PLGA formulation stability here:
👉 https://resolvemass.ca/plga-formulation-stability/
SUMMARY
- PLGA Degradation Profile governs how rapidly and predictably poly(lactic-co‑glycolic acid) (PLGA) breaks down in biological environments — setting the stage for drug release, structural stability, and safety of PLGA‑based formulations.
- The rate and pattern of the PLGA Profile depend on factors such as polymer composition (lactide:glycolide ratio), molecular weight, end‑group chemistry, crystallinity, pH, temperature, and presence of catalysts or enzymes.
- A well-characterized PLGA Profile ensures controlled and reproducible drug release kinetics, improving dosing precision and therapeutic outcomes.
- Conversely, unpredictable or poorly optimized PLGA Profile can lead to burst release, incomplete drug release, drug degradation, local acidity, inflammation, or instability — potentially compromising safety and efficacy.
- Understanding and optimizing the PLGA Profile enhances drug stability, maintains safety margins, preserves bioactivity, and supports regulatory compliance and product quality — which are essential to the mission of ResolveMass Laboratories Inc.
1: UNDERSTANDING THE PLGA DEGRADATION PROFILE
What is the PLGA Degradation Profile?
The PLGA Degradation Profile describes the temporal and mechanistic pattern by which PLGA undergoes hydrolytic (and sometimes enzymatic) cleavage, converting from polymer chains to oligomers, lactic and glycolic acids, and eventually to carbon dioxide and water. This degradation trajectory — including onset, rate, and by‑products — defines the profile.
Key Characteristics of a PLGA Degradation Profile
- Onset of degradation: when hydrolysis begins significantly.
- Degradation rate: how fast polymer molecular weight declines.
- Degradation phases: lag phase, bulk degradation, erosion phase.
- By‑product profile: accumulation of acidic monomers, oligomers, pH change.
A well-defined PLGA Profile allows drug developers to predict when and how the drug will be released, how the polymer matrix will change, and what by‑products will accumulate.
To understand how monomer ratio affects degradation, see our NMR-based characterization article:
👉 https://resolvemass.ca/nmr-spectroscopy-for-accurate-monomer-ratio/
2: FACTORS INFLUENCING PLGA DEGRADATION PROFILE IN DRUG FORMULATIONS
Why does the PLGA Degradation Profile vary with formulation parameters?
Depending on formulation design and manufacturing choices, the PLGA Degradation Profile may shift dramatically. Below are the most influential factors:
| Factor | Impact on PLGA Degradation Profile |
|---|---|
| Lactide:Glycolide Ratio | Higher glycolide content → faster degradation; more lactide → slower degradation. |
| Molecular Weight (Mw) | Higher Mw → slower degradation (longer chains take more time to cleave). |
| Polymer End Groups (acid vs ester capped) | Acid‑terminated PLGA → faster degradation due to autocatalysis. |
| Crystallinity / Glass Transition Temperature (Tg) | More crystalline or higher Tg → slower water penetration → slower degradation. |
| pH and Buffering Capacity of Medium | Lower pH / poor buffering → accelerated degradation; acidic by‑products can further catalyze degradation. |
| Temperature and Storage Conditions | Elevated temperature → faster degradation or pre‑degradation; cold/humid storage may affect stability. |
| Presence of Catalysts / Enzymes / Salts | Salts, enzymes, or certain excipients may accelerate hydrolysis or influence degradation pathways. |
| Drug/Polymer Interactions | Acidic or basic drugs may catalyze or inhibit degradation, altering the PLGA Degradation Profile and release kinetics. |
Because of these variables, each PLGA formulation requires careful evaluation of its unique PLGA Profile during development.
Learn how end-capped PLGA affects degradation:
👉 https://resolvemass.ca/end-capped-plga/
And how to dissolve PLGA safely in solvents during formulation:
👉 https://resolvemass.ca/dissolving-plga-in-solvents/
3: IMPACT OF DEGRADATION PROFILE ON DRUG RELEASE KINETICS
How does PLGA Degradation Profile affect drug release behavior?
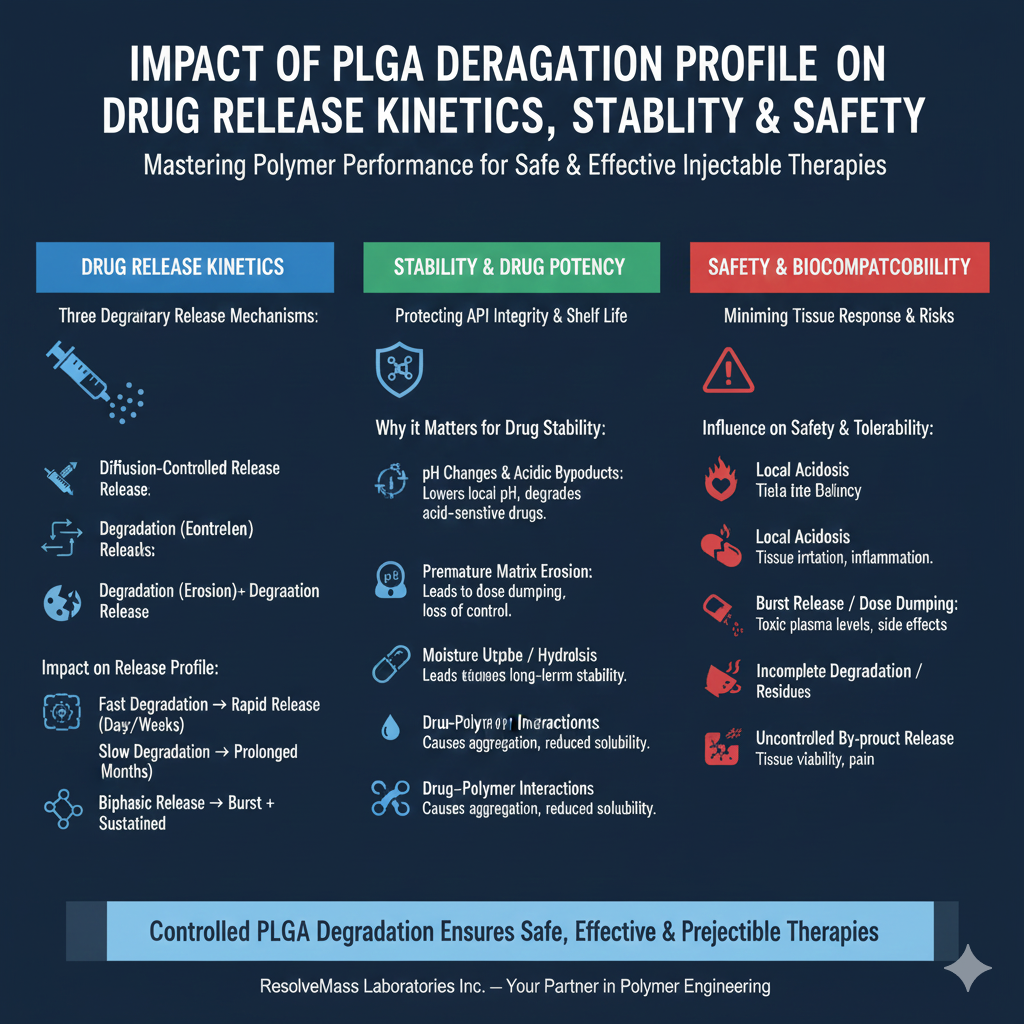
The PLGA Profile directly shapes how and when a drug is released from PLGA-based systems. There are three primary release mechanisms influenced by degradation:
- Diffusion-controlled release — Early release phase where the drug diffuses out before substantial PLGA degradation.
- Degradation (erosion)-controlled release — As PLGA polymer chains cleave, the matrix erodes, releasing the entrapped drug.
- Combined diffusion + degradation release — Most typical in PLGA systems: an initial diffusion phase, followed by a degradation‑driven release phase.
A predictable PLGA Profile allows designers to tailor the release profile — for example, achieving a sustained release over several weeks or a pulsatile release over months.
For examples of practical drug release optimization strategies, explore our custom release engineering article:
👉 https://resolvemass.ca/custom-plga-release-control/
PLGA degradation also plays a major role in depot injections. Learn more:
👉 https://resolvemass.ca/plga-depot-formulation/
Typical Scenarios of PLGA Degradation Profile and Drug Release
- Fast degradation profile → rapid drug release (days to weeks), useful for short‑term therapy.
- Slow degradation profile → prolonged release (weeks to months), ideal for long‑acting injectables or implants.
- Biphasic release → initial burst (diffusion) + slow sustained release (degradation), useful when loading doses followed by maintenance therapy.
At ResolveMass Laboratories Inc., understanding and controlling PLGA Profile enables us to optimize drug release for specific therapeutic goals, ensuring consistency batch to batch.
4: IMPACT OF PLGA DEGRADATION PROFILE ON STABILITY AND DRUG POTENCY
Why does PLGA Degradation Profile matter for drug stability?
The degradation of PLGA affects stability in multiple ways — from physical integrity of the dosage form to chemical stability and bioactivity of the active pharmaceutical ingredient (API). Here’s how:
- pH Changes and Acidic By‑products: As PLGA degrades, lactic and glycolic acids accumulate. Without adequate buffering or design, this may lower local pH — potentially degrading acid‑sensitive drugs, reducing potency or causing chemical modifications.
- Premature Matrix Erosion: If PLGA Degradation Profile is faster than expected (e.g. due to poor polymer selection or storage conditions), the matrix may erode before intended shelf‑life or dosing interval — leading to dose dumping or loss of controlled release.
- Moisture Uptake / Hydrolysis during Storage: Exposure to moisture or humidity may initiate hydrolysis even before administration — compromising long‑term stability.
- Drug–Polymer Interactions: Some drugs may interact with acidic degradation products — leading to API precipitation, aggregation, or reduced solubility.
Therefore, a carefully characterized PLGA Profile under realistic storage conditions is essential to ensure that the final drug product remains stable, potent, and safe throughout shelf life and use.
For formulation scientists working on reference drug comparison, we provide a deep dive into PLGA characterization for RLD development:
👉 https://resolvemass.ca/plga-characterization-for-rld/
Also see how Q1/Q2 polymer equivalence assessment ensures degradation consistency across batches:
👉 https://resolvemass.ca/q1-q2-polymer-equivalence-assessment/
5: IMPACT OF PLGA DEGRADATION PROFILE ON SAFETY AND BIOCOMPATIBILITY
How does PLGA Degradation Profile influence safety and tolerability?
Safety of PLGA-based drug delivery systems is closely tied to the degradation behavior of the polymer — because degradation by‑products and the kinetics of release may affect tissue response and systemic exposure.
- Local Acidosis: Rapid degradation may lead to accumulation of lactic and glycolic acid, lowering local pH; this can irritate tissue, cause inflammation, or affect surrounding cells.
- Burst Release / Dose Dumping Risks: If PLGA Degradation Profile is not controlled, there can be an unintended burst release — leading to toxic plasma levels or side effects.
- Incomplete Degradation / Residues: Poorly designed PLGA (e.g. high crystallinity) may degrade very slowly, leading to polymer residue persisting in tissues for extended periods, potentially triggering foreign‑body reaction.
- Uncontrolled By‑product Release: Rapid pH drop or acidic microenvironment may impact surrounding tissue viability or cause pain, fibrosis, or impaired healing.
At ResolveMass Laboratories Inc., we emphasize rigorous in‑vitro and in‑vivo testing of PLGA Profile to assess local and systemic safety — providing clients robust data before clinical development.
PLGA safety considerations are especially important in oncology drug delivery. Read more here:
👉 https://resolvemass.ca/plga-oncology-formulation/
6: DESIGN STRATEGIES TO OPTIMIZE PLGA DEGRADATION PROFILE FOR DESIRED OUTCOMES
How can formulation scientists control the PLGA Degradation Profile?
Based on our extensive formulation experience, here are key design strategies:
- Selecting Appropriate Lactide:Glycolide Ratio
• Use lactide‑rich PLGA for slower degradation (long-term release).
• Use glycolide‑rich PLGA for faster degradation (short-term release). - Choosing Suitable Molecular Weight & End‑Group Chemistry
• Higher molecular weight & ester-capped → slower degradation.
• Lower molecular weight & acid-capped → faster degradation. - Controlling Polymer Crystallinity and Tg
• Use PLGA grades with lower crystallinity or glassy amorphous structure to facilitate homogeneous degradation. - Incorporating pH‑buffering excipients or basic salts
• Buffer acidity from degradation (e.g. incorporate basic salts, buffering agents) to protect acid‑sensitive drugs and local tissues. - Optimizing Polymer–Drug Ratio and Loading
• Avoid overloading drug to minimize drug–polymer interactions and acidic microclimate build-up. - Ensuring Moisture‑Controlled Storage and Manufacturing Environment
• Dry manufacturing under controlled humidity; use desiccants; package to prevent moisture uptake. - In‑vitro and Accelerated Degradation Testing
• Simulate physiological and storage conditions; monitor degradation kinetics, pH, by‑products, drug release, and stability.
By employing these strategies, scientists can shape the PLGA Profile to match therapeutic goals — whether for rapid release vaccines or long‑acting implants.
If you need help designing reproducible, scalable PLGA systems, review our scale-up guidance:
👉 https://resolvemass.ca/plga-scale-up-case-study/
👉 https://resolvemass.ca/plga-microencapsulation-scale-up/
👉 https://resolvemass.ca/plga-microsphere-case-study/
7: CASE STUDIES: EXAMPLES OF PLGA DEGRADATION PROFILE APPLICATIONS
Long‑Acting Injectable for Chronic Disease
A PLGA formulation with a 75:25 lactide:glycolide ratio, high molecular weight, and ester end‑caps demonstrates a slow PLGA Profile — releasing drug steadily over 3–6 months. This ensures stable therapeutic levels, reduces dosing frequency, and improves patient compliance. Stability testing shows minimal acidic by‑product accumulation — preserving drug potency and minimizing tissue irritation.
Vaccine Delivery with Controlled Burst + Sustained Release
A PLGA microparticle with 50:50 ratio and acid‑terminated low‑Mw polymer yields a “biphasic” PLGA Profile: an initial burst of antigen release (to prime immune response), followed by slow sustained release (for boosting) — optimizing immunogenicity while maintaining safety.
Both examples are aligned with manufacturing strategies described here:
👉 https://resolvemass.ca/plga-microencapsulation-scale-up/
8: CHALLENGES AND MITIGATION STRATEGIES RELATED TO PLGA DEGRADATION PROFILE
Common Challenges When PLGA Degradation Profile Is Not Properly Managed
- Unintended Burst Release or Dose Dumping — leading to safety risks.
- Drug Degradation Due to Acidic Microenvironment — loss of potency or altered pharmacodynamics.
- pH‑Induced Tissue Toxicity or Irritation — local inflammation or discomfort.
- Batch‑to‑Batch Variability — inconsistent PLGA Degradation Profile may compromise reproducibility and regulatory compliance.
- Storage Stability Issues — premature polymer degradation during storage.
Mitigation Strategies
- Use buffered formulations or basic excipients to neutralize acidic by‑products.
- Conduct rigorous accelerated stability testing under stress conditions.
- Standardize polymer sourcing: use pharmaceutical‑grade PLGA with tight specifications.
- Perform in‑vitro release and degradation profile characterization early in development.
- Monitor degradation by‑products, pH changes, and residual polymer in preclinical safety studies.
For deep insight into PLGA behavior in real-world conditions, explore our characterization resources:
👉 https://resolvemass.ca/plga-characterization-for-rld/
👉 https://resolvemass.ca/nmr-spectroscopy-for-accurate-monomer-ratio/
9: WHY PLGA DEGRADATION PROFILE MATTERS FOR REGULATORY COMPLIANCE AND QUALITY CONTROL
Regulatory agencies expect robust data demonstrating that polymer‑based drug delivery systems are safe, stable, and reproducible. A well-characterized PLGA Profile supports:
- Predictable drug release kinetics — enabling accurate dosing and labeling.
- Demonstrated stability throughout shelf‑life and use.
- Safety profile — minimizing risk of local irritation or toxicity.
- Batch consistency — reducing variability in degradation rate and release behavior.
- Transparency for regulatory submission — supporting quality, safety, and efficacy documentation.
At ResolveMass Laboratories Inc., we integrate PLGA Profile studies — including polymer characterization, in‑vitro degradation, drug release testing, by‑product analysis, and stability — as standard practice in our formulation and quality workflows.
Learn more:
👉 https://resolvemass.ca/q1-q2-polymer-equivalence-assessment/
CONCLUSION
In summary, the PLGA Degradation Profile is a cornerstone attribute for any PLGA‑based drug delivery system. It dictates how the polymer degrades, when and how the drug is released, how stable the formulation remains, and how safe the product is for patients. By thoroughly understanding and optimizing the PLGA Profile — from polymer selection to formulation design, storage conditions, and release testing — developers can create high‑quality, safe, stable, and effective drug products.
At ResolveMass Laboratories Inc., we bring deep expertise in polymer science, drug formulation, and quality assurance to deliver PLGA-based products with reliable, predictable PLGA Profile — ready for preclinical and clinical development with confidence.
FAQs on PLGA Degradation Affects Drug Release, Safety, and Stability
The PLGA Degradation Profile describes how PLGA undergoes hydrolysis over time, breaking down into lactic and glycolic acids. This degradation pathway determines how fast the polymer loses molecular weight, when the matrix begins eroding, and how the drug is released from the system.
A complete PLGA Degradation Profile includes lag phase, bulk degradation phase, erosion phase, and the accumulation of acidic by-products. Understanding this profile enables scientists to predict release kinetics and ensure formulation stability and safety.
PLGA degradation controls whether release is diffusion-driven, erosion-controlled, or biphasic.
A faster PLGA Degradation Profile (e.g., with low molecular weight, 50:50 ratio, acid end-caps) accelerates polymer erosion and produces rapid release.
A slow PLGA Degradation Profile (e.g., high Mw, lactide-rich, ester-capped) supports extended release over weeks–months.
Drug release is tightly linked to the rate of chain scission and porosity formation, making degradation profiling essential.
A higher glycolide content increases hydrophilicity, accelerates water penetration, and produces a faster PLGA Degradation Profile.
A higher lactide content makes the polymer more hydrophobic and crystalline, resulting in slower degradation.
Common examples:
-50:50 PLGA → fastest degradation
-75:25 or 85:15 PLGA → moderate to slow
-100% PLA → slowest, often months to years
Selecting the correct ratio is one of the strongest levers for tuning the degradation profile.
End-capping determines how easily the polymer undergoes autocatalysis during hydrolysis.
-Acid-terminated PLGA contains free –COOH groups that catalyze chain scission, creating a faster PLGA Degradation Profile.
-Ester-capped PLGA lacks acidic end-groups and therefore resists hydrolysis, providing a slower, more controlled degradation profile.
End-group control is essential for long-acting injectables, implants, and depot formulations.
To achieve extended release over months, scientists may use:
-Higher molecular weight PLGA
-Lactide-rich or PLA-rich polymers
-Ester end capping
-Reduced porosity and tougher matrices
-Hydrophobic excipients
-Controlled microencapsulation techniques
-Moisture-free manufacturing environments
These strategies ensure the polymer remains stable and degrades gradually rather than collapsing rapidly.
Reference
- Makadia HK, Siegel SJ. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. J Control Release. 2011;155(2):159–165.https://doi.org/10.1016/j.jconrel.2011.02.010
- Gentile P, Chiono V, Carmagnola I, Hatton PV. An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int J Mol Sci. 2014;15(3):3640–3659.https://doi.org/10.3390/ijms15033640

