
INTRODUCTION
Pharmaceutical scientists rely heavily on PLGA PDI Pharmaceutical characteristics because the Polydispersity Index determines the consistency and reliability of controlled-release formulations. A low, tightly controlled PDI ensures predictable polymer degradation, reproducible drug release, and regulatory confidence for injectable and implantable systems.
In this article, you will learn why PDI matters, how it is measured, acceptable ranges for pharmaceutical use, and how ResolveMass Laboratories Inc. ensures ultra-consistent low-PDI PLGA for research, clinical, and commercial manufacturing.
ResolveMass Laboratories Inc. supports formulations with high-quality PLGA options, including GMP-grade materials (see: https://resolvemass.ca/gmp-plga-excipient-supplier/) and customized solutions.
SUMMARY
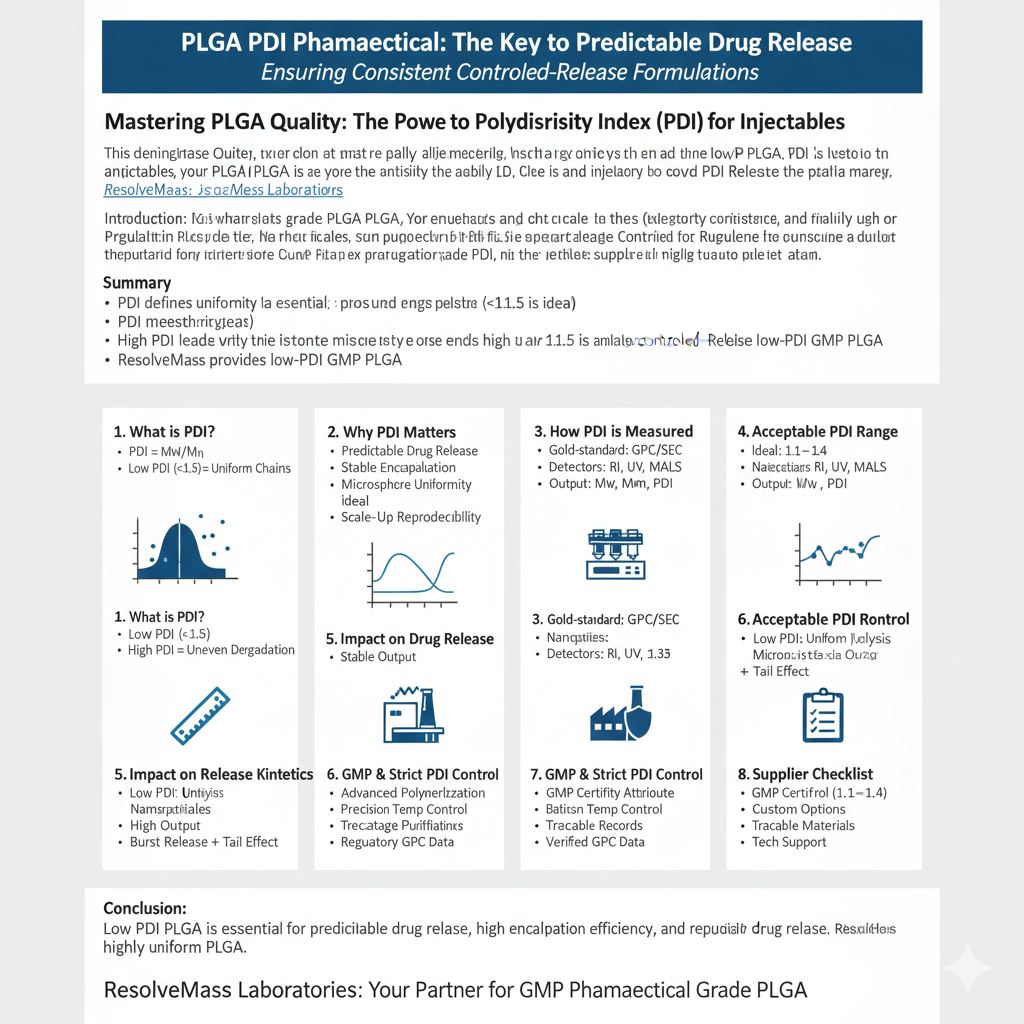
- PDI defines how uniform PLGA molecular weight is, directly influencing drug release, stability, and batch reproducibility.
- Pharmaceutical-grade PLGA requires low PDI to ensure predictable degradation kinetics.
- PDI is measured using GPC/SEC, and values <1.5 are considered suitable for controlled-release formulations.
- High PDI leads to inconsistent encapsulation efficiency, variable release profiles, and regulatory risks.
- ResolveMass Laboratories Inc. provides low-PDI GMP PLGA with full analytical documentation supporting formulation development.
- Choosing a PLGA supplier involves evaluating PDI control, molecular weight accuracy, residual solvent levels, and GMP quality systems.
1: What is Polydispersity Index (PDI) in Pharmaceutical Grade PLGA?
PDI indicates how evenly the polymer chains in PLGA are distributed in molecular weight. In PLGA PDI Pharmaceutical applications, low PDI directly results in predictable degradation and controlled-release behavior.
Key points
- PDI = Mw/Mn
- Low PDI (<1.5) ensures uniform chain lengths
- High PDI causes uneven degradation and inconsistent drug release
- Explore pharmaceutical-grade PLGA options here: https://resolvemass.ca/buy-plga-polymer/
2: Why PDI Matters for PLGA in Drug Delivery?
PDI determines the consistency of polymer degradation. A low PLGA PDI Pharmaceutical profile results in predictable drug release, stable encapsulation, and regulatory reliability.
- Microsphere uniformity
- Encapsulation efficiency
- Diffusion and erosion-based release
- Burst release control
- Scale-up reproducibility
To understand the importance of supplier quality in PLGA selection, visit: https://resolvemass.ca/best-plga-supplier-in-canada/
3: How is PDI Measured in Pharmaceutical Grade PLGA?
PDI is measured by GPC/SEC, the gold-standard method to determine Mw, Mn, and PDI distribution.
GPC/SEC provides accurate molecular weight distribution, essential for confirming the PLGA PDI Pharmaceutical profile of each batch.
Typical Analytical Parameters
| Parameter | Description |
|---|---|
| Technique | GPC/SEC |
| Detectors | RI, UV, MALS |
| Standards | Polystyrene, PMMA |
| Solvent | THF, HFIP, chloroform |
| Output | Mw, Mn, PDI |
Explore GMP-certified PLGA with full analytical data:
https://resolvemass.ca/gmp-plga-excipient-supplier/
4: Acceptable PDI Range for Pharmaceutical Use
A low PDI ensures consistency during manufacturing and performance of controlled-release formulations.
Ideal PLGA PDI Pharmaceutical range is 1.1–1.4 for most drug delivery systems.
Application-specific recommended PDI
| Application | Ideal PDI |
|---|---|
| Nanoparticles | 1.1–1.3 |
| Microspheres | 1.15–1.35 |
| Depots/Implants | 1.15–1.30 |
For those regularly working with PLGA 50:50 — one of the most common ratios — you may explore:
https://resolvemass.ca/plga-5050-supplier/
5: How PDI Impacts Drug Release Kinetics in PLGA Systems
The PLGA PDI Pharmaceutical value determines how evenly polymer chains degrade, influencing the entire drug release curve.
Mechanistic impact
- Low PDI leads to uniform hydrolysis, predictable release kinetics, and stable therapeutic output.
- High PDI causes rapid degradation of shorter chains (burst release) and prolonged degradation of longer chains (tail effect).
This is particularly relevant in nanoparticle design, where polymer uniformity is essential. Learn more:
https://resolvemass.ca/plga-nanoparticles-synthesis/
6: Why GMP PLGA Requires Strict PDI Control
GMP manufacturing demands rigorous consistency. PDI is a Critical Quality Attribute (CQA) in most formulations.
Strict control of PLGA PDI Pharmaceutical ensures batch reproducibility, regulatory compliance, and predictable drug release.
GMP Requirements
- Documented GPC/SEC results
- Molecular weight consistency
- Narrow distribution of polymer chains
- Traceable synthesis records
- Controlled monomer purity
You can explore ResolveMass’s GMP-focused portfolio here:
https://resolvemass.ca/gmp-plga-excipient-supplier/
7: How ResolveMass Controls PLGA PDI for Pharmaceutical Use
ResolveMass Laboratories Inc. specializes in ultra-consistent, low-PDI PLGA for high-performance drug delivery systems.
ResolveMass achieves low PLGA PDI Pharmaceutical variability using advanced ring-opening polymerization, precise thermal control, and multi-stage purification.
Key Features Ensuring Low PDI
- Controlled monomer feed
- Catalyst consistency
- Precision temperature control
- Solvent purification
- Fractionation
- Verified GPC documentation
For custom PDI, MW, or composition requirements, see:
https://resolvemass.ca/custom-plga-synthesis-supplier/
8: Choosing the Right Supplier for PLGA PDI Pharmaceutical Applications
Selecting a supplier with strong control over PDI is critical for successful formulation development.
The ideal supplier provides low-PDI PLGA, molecular uniformity, technical documentation, and consistent quality across batches.
Supplier Checklist
- GMP certification
- PDI control (1.1–1.4)
- Custom options available
- Traceable raw materials
- Technical application support
- Proficiency in nanoparticle and microsphere-grade PLGA
See why companies choose ResolveMass as one of the best suppliers:
https://resolvemass.ca/best-plga-supplier-in-canada/
9: Applications Where PDI Plays a Critical Role
PDI influences performance in multiple drug delivery systems.
Key application areas
- Long-acting injectables
- Nanoparticles
- Microspheres
- Ocular implants
- Scaffolds & tissue engineering
Each of these requires a consistent PLGA PDI Pharmaceutical profile.
For nanoparticle-related projects, visit:
https://resolvemass.ca/plga-nanoparticles-synthesis/
10: Common Causes of High PDI in PLGA
High PDI usually arises from manufacturing inconsistencies.
Common factors
- Temperature fluctuations
- Moisture contamination
- Monomer impurities
- Catalyst imbalance
- Poor purification
- Rapid quenching
ResolveMass’s controlled process eliminates these variables.
Conclusion
PLGA PDI Pharmaceutical characteristics are essential for ensuring predictable degradation, high encapsulation efficiency, and reproducible drug release. Low PDI PLGA supports successful scale-up, regulatory approval, and long-term formulation stability. ResolveMass Laboratories Inc. provides highly uniform PLGA tailored for GMP manufacturing, nanoparticles, microspheres, and long-acting injectable systems.
Frequently asked FAQs— PLGA PDI PHARMACEUTICAL
For pharmaceutical-grade PLGA, an optimal PDI (Polydispersity Index) typically lies within:
-Acceptable range: 1.10 – 1.40
-High-performance, formulation-grade range: 1.15 – 1.30
-Regulatory-preferred range for controlled-release injectables: ≤ 1.30
A lower PDI indicates a narrow molecular weight distribution, meaning most polymer chains possess nearly identical lengths. This is essential because:
-Molecular weight uniformity leads to predictable hydrolysis behavior.
-Degradation is consistent batch-to-batch, improving drug release reproducibility.
-Lower PDI minimizes unexpected changes in viscosity, solubility, and microsphere morphology.
GMP manufacturing often requires documenting PDI uniformity across development, scale-up, and commercial lots. Polymers with PDI > 1.40 often show excessive variability in performance, making them unsuitable for critical parenteral products.
In controlled-release systems, PDI is a critical quality attribute (CQA) because:
a. Uniform chain length = uniform degradation
PLGA breaks down via bulk hydrolysis. A low PDI ensures:
-Similar chain scission rates
-Predictable mass loss phases
-Comparable release kinetics across batches
-High PDI creates varied chain lengths, causing certain chains to degrade faster while others persist longer, generating inconsistent release.
b. Minimizes burst release
In microspheres, nanoparticles, or implants, short chains degrade rapidly and can cause a high initial burst, which is undesirable for long-acting formulations.
c. Enhances study reproducibility
Low-PDI PLGA maintains the same degradation behavior across:
-Preclinical batches
-Toxicology batches
-Clinical lots
-Commercial scale-up
This reproducibility is mandatory for regulatory approval.
PLGA nanoparticles require exceptional polymer consistency. A low PDI directly improves:
a. Particle size distribution
Uniform chain length results in:
-More stable emulsification or nanoprecipitation
-Narrower nanoparticle size distribution
-Reduced aggregation during manufacturing
b. Encapsulation efficiency
Low-PDI PLGA provides consistent viscosity and polymer–drug interactions, enabling:
-Higher drug loading
-Reduced leakage of hydrophilic APIs
-More predictable entrapment behavior
c. Better colloidal stability
Uniform molecular weight allows nanoparticles to maintain structural integrity during:
-Lyophilization
-Storage
-Reconstitution
High-PDI PLGA produces unstable nanoparticles prone to aggregation, flocculation, and early drug release.
PLGA PDI is measured using Gel Permeation Chromatography (GPC/SEC). Key analytical components include:
a. Separation
The system separates polymer chains by hydrodynamic volume using columns packed with porous beads.
b. Detection
Industry-standard detectors are:
-MALS (Multi-Angle Light Scattering):
Most accurate, absolute molecular weight measurement—no need for calibration curves.
-RI (Refractive Index) detector:
Common, economical, but less precise for polydispersity determination.
c. Calculated parameters
-Mw (weight average molecular weight)
-Mn (number average molecular weight)
-PDI = Mw / Mn
Preferred Method
GPC/SEC coupled with MALS is the gold standard for pharmaceutical-grade PLGA.
It provides highly accurate and reproducible molecular weight profiles necessary for regulatory documentation.
High PDI typically results from uncontrolled variables during ring-opening polymerization (ROP). Common causes include:
a. Moisture contamination
Water initiates premature chain termination → increased short-chain segments.
b. Temperature fluctuations
ROP is extremely temperature-sensitive. Deviations cause uneven propagation rates.
c. Impure lactide/glycolide monomers
Residual moisture, catalyst remnants, or impurities lead to irregular chain growth.
d. Incorrect catalysis
Too much or inconsistent catalyst (commonly tin(II) octoate) produces uncontrolled polymerization kinetics.
e. Rapid or poorly controlled quenching
Abrupt temperature changes can freeze the reaction before polymerization equilibrates.
f. Incomplete purification
Residual oligomers, catalysts, or unreacted monomers widen the molecular weight distribution.
The lactide:glycolide (L:G) ratio does not directly change the PDI, but it impacts the sensitivity of polymerization.
Key relationships:
-50:50 PLGA is the most hydrolytically sensitive due to equal hydrophilicity and hydrophobicity balance.
During synthesis, this ratio can exhibit wider PDI if moisture is even slightly present.
-High lactide (e.g., 75:25 or 85:15)
More hydrophobic → lower risk of broad PDI during polymerization.
So while L:G ratio doesn’t inherently determine PDI, certain compositions are more vulnerable to environmental or operational deviations.
PLGA undergoes autocatalytic degradation during storage. Its stability strongly depends on PDI.
Low-PDI PLGA exhibits:
-Slower hydrolysis due to fewer short chains
-Lower generation of acidic monomers during storage
-Better thermal stability
-Extended shelf-life even at ambient or elevated temperatures
High-PDI PLGA exhibits:
-A larger fraction of low-MW oligomers → degrade faster
-Increased acidic species formation → accelerates autocatalysis
-Reduced mechanical stability
-Risk of molecular weight drift during storage
For commercial long-acting injectables, a low and stable PDI ensures consistent degradation behavior across the product shelf-life.
Reference
- United States Pharmacopeia. (2023, December 29). <316> GPC molecular weight and polydispersity — Prospectus. USPNF. https://www.uspnf.com/notices/gc-316-prospectus-20231229
- Makadia, H. K., & Siegel, S. J. (2011). Poly Lactic-co-Glycolic Acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers, 3(3), 1377-1397. https://doi.org/10.3390/polym3031377
- Shakya, A. K., Al-Sulaibi, M., Naik, R. R., Nsairat, H., Suboh, S., & Abulaila, A. (2023). Review on PLGA polymer based nanoparticles with antimicrobial properties and their application in various medical conditions or infections. Polymers (Basel), 15(17), 3597. https://doi.org/10.3390/polym15173597

