
INTRODUCTION
PLGA Residual Solvents are trace organic chemicals remaining after polymer synthesis, and they significantly influence the safety, quality, performance, and regulatory acceptability of PLGA for injectable and implantable drug delivery. Because PLGA is widely used in controlled-release systems, long-acting injectables, nanoparticles, depot formulations, and oncology formulations, managing PLGA Residual Solvents is one of the most critical aspects of polymer qualification.
To support advanced formulations, ResolveMass Laboratories Inc. offers pharmaceutical-grade PLGA with ultra-low solvent content and deep analytical characterization, as detailed throughout this article.
For background on how PLGA influences drug delivery systems, see:
- PLGA for Depot Formulation: https://resolvemass.ca/plga-for-depot-formulation/
- PLGA for Controlled Release: https://resolvemass.ca/plga-for-controlled-release/
SUMMARY
- PLGA Residual Solvents directly impact polymer performance, drug release, stability, and regulatory acceptance—making ICH Q3C compliance essential.
- The most relevant solvents in PLGA manufacturing include DCM, acetone, ethyl acetate, acetonitrile, and THF.
- Testing of PLGA Residual Solvents is performed using validated GC-FID, HS-GC, GC-MS, and Karl Fischer methods.
- Reliable suppliers must provide CoA solvent data, GMP-ready documentation, validation studies, and batch reproducibility evidence.
- ResolveMass Laboratories Inc. supports formulations through custom PLGA synthesis, nanoparticle development, depot formulation, and scale-up case studies.
1: WHAT ARE RESIDUAL SOLVENTS IN PLGA?
Residual solvents are organic compounds used during polymerization or purification that remain trapped within the PLGA matrix. They must be tightly controlled because PLGA Residual Solvents influence drug release kinetics, polymer stability, microsphere porosity, and patient safety.
Common solvents in PLGA synthesis
| Solvent | Application | ICH Class | Limit |
|---|---|---|---|
| Dichloromethane (DCM) | Polymerization, dissolution | Class 2 | 600 ppm |
| Acetone | Purification | Class 3 | 5000 ppm |
| Ethyl Acetate | Washing | Class 3 | 5000 ppm |
| Acetonitrile | Specialty polymerization | Class 2 | 410 ppm |
| THF | Co-solvent | Class 2 | 720 ppm |
To understand solvent–polymer interactions, see:
- Dissolving PLGA in Solvents: https://resolvemass.ca/dissolving-plga-in-solvents/
2: WHY PLGA RESIDUAL SOLVENTS MATTER FOR FORMULATION QUALITY
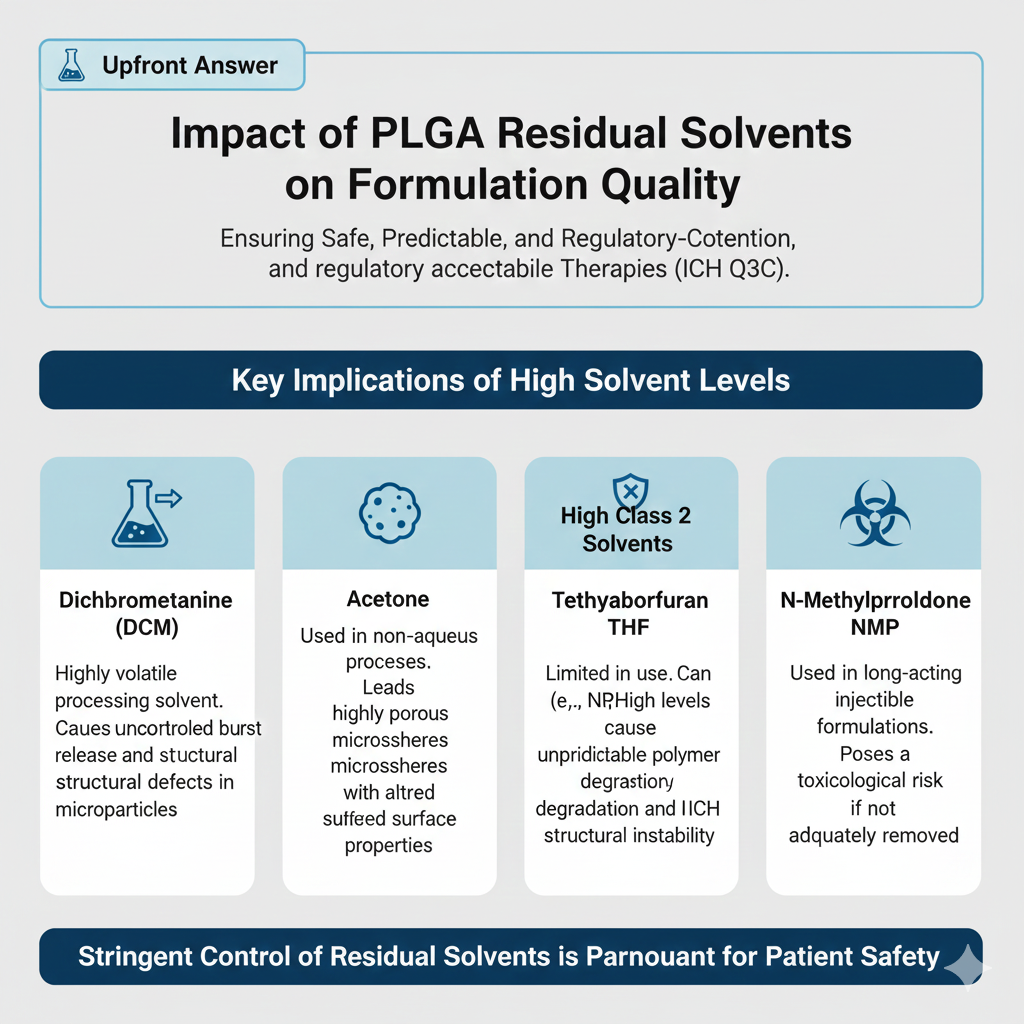
Upfront answer: PLGA Residual Solvents impact microsphere architecture, burst release, molecular weight retention, and regulatory acceptability.
Key implications
- High DCM → uncontrolled burst release
- High acetone → porous microspheres
- High Class 2 solvents → regulatory rejection
- High THF → unpredictable polymer degradation
- High NMP → toxicological risk
Deep technical study:
- PLGA Microencapsulation Scale-Up: https://resolvemass.ca/plga-microencapsulation-scale-up/
- PLGA Nanoparticles Synthesis: https://resolvemass.ca/plga-nanoparticles-synthesis/
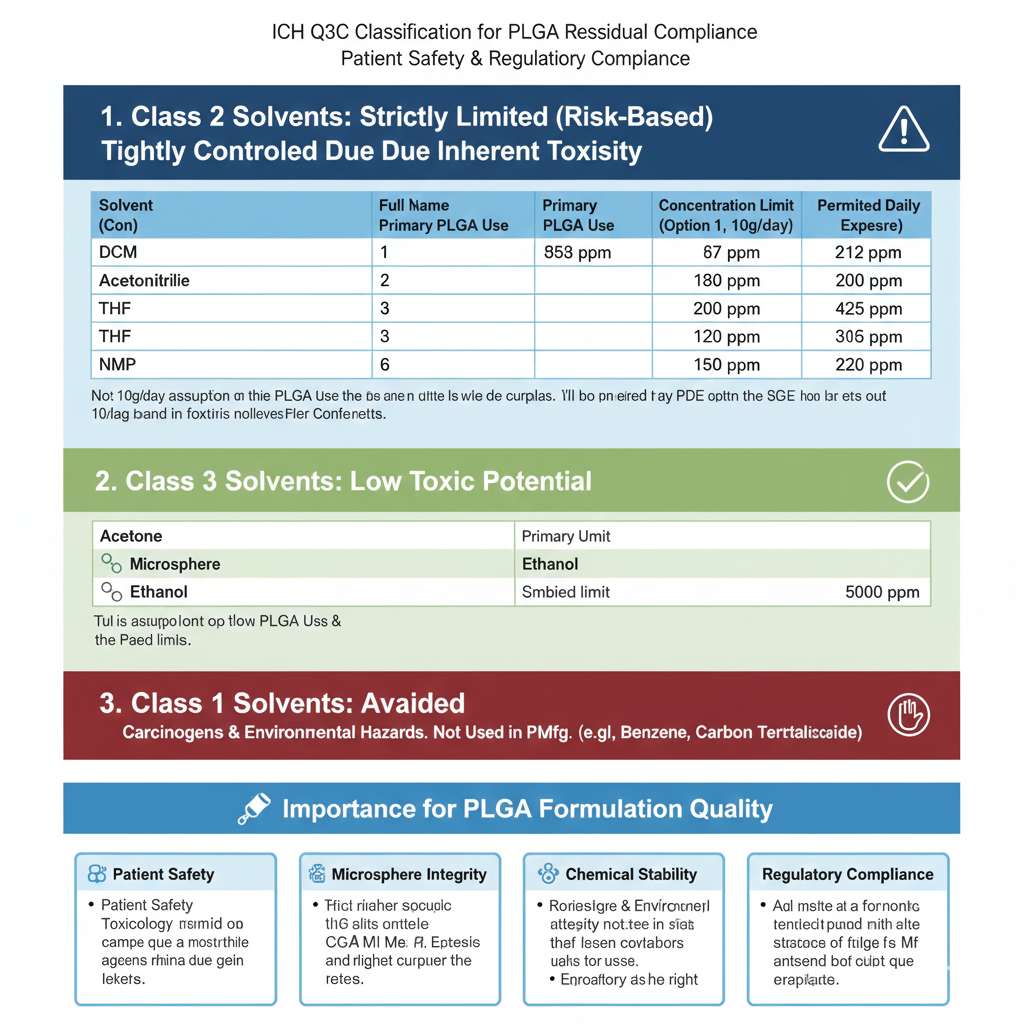
3: ICH Q3C CLASSIFICATION FOR PLGA RESIDUAL SOLVENTS
ICH Class 2 – Strictly Regulated Solvents in PLGA
PLGA Residual Solvents in Class 2 must be minimized due to toxicity.
Includes:
- DCM
- Acetonitrile
- THF
- NMP
ICH limits range between 410–720 ppm.
Helpful formulation references:
- PLGA Characterization for RLD: https://resolvemass.ca/plga-characterization-for-rld/
- Q1/Q2 Polymer Equivalence: https://resolvemass.ca/q1-q2-polymer-equivalence-assessment/
ICH Class 3 – Lower-Risk Solvents
Includes acetone, ethyl acetate, IPA.
Though allowed up to 5000 ppm, good PLGA suppliers maintain much lower levels.
Related resource:
- PLGA Formulation Stability: https://resolvemass.ca/plga-formulation-stability/
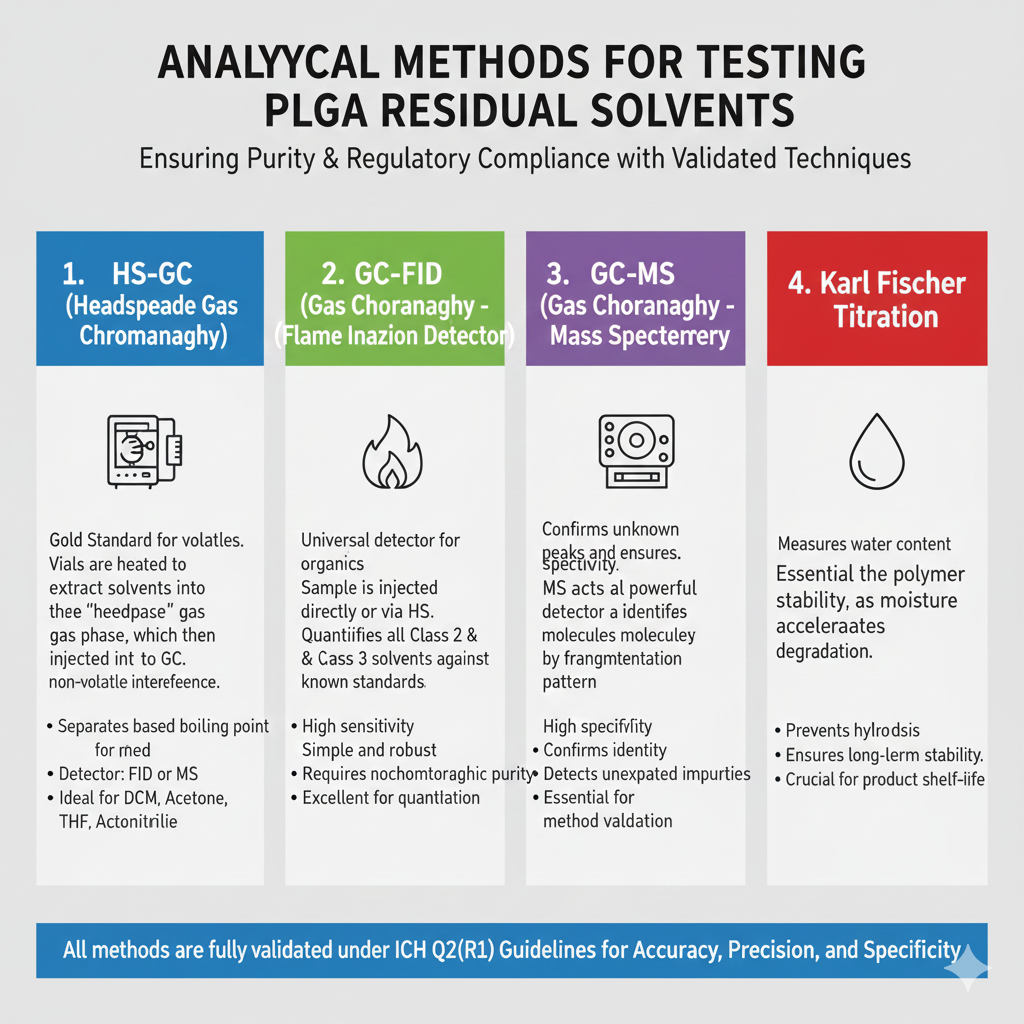
4: ANALYTICAL METHODS FOR TESTING PLGA RESIDUAL SOLVENTS
PLGA Residual Solvents are measured using validated methods such as GC-FID, GC-MS, and HS-GC under ICH Q2 standards.
Key analytical approaches:
- HS-GC (Headspace Gas Chromatography)
- Gold standard for volatile solvents like DCM
- GC-FID
- Quantifies all Class 2 & Class 3 solvents
- GC-MS
- Confirms unknown peaks
- Karl Fischer
- Moisture analysis ensuring polymer stability
For understanding monomer ratio characterization and polymer analytics:
- NMR Spectroscopy for Accurate Monomer Ratio
https://resolvemass.ca/nmr-spectroscopy-for-accurate-monomer-ratio/ - PLGA Molecular Weight & PDI
https://resolvemass.ca/plga-polymer-molecular-weight-and-pdi/ - Determining Molecular Weight of PLGA
https://resolvemass.ca/how-to-determine-molecular-weight-of-plga-polymers/
Related PLGA Analytical Resources
- https://resolvemass.ca/plga-microsphere-case-study/
- https://resolvemass.ca/custom-plga-release-control/
5: SUPPLIER REQUIREMENTS FOR LOW-SOLVENT PLGA
A compliant PLGA supplier must provide validated data, ICH-aligned CoAs, batch reproducibility, and GMP manufacturing assurance.
Supplier qualifications checklist
- GMP and pharmaceutical-grade PLGA
- Batch-specific CoA listing residual solvents
- Method validation studies
- In-process solvent removal controls
- Long-term stability data
ResolveMass provides GMP-ready PLGA for global developers:
- Pharmaceutical-Grade PLGA Supplier
https://resolvemass.ca/pharmaceutical-grade-plga-supplier/ - GMP PLGA Excipient Supplier
https://resolvemass.ca/gmp-plga-excipient-supplier/ - PLGA Supplier Canada
https://resolvemass.ca/plga-supplier-canada/ - PLGA Supplier United States
https://resolvemass.ca/plga-supplier-united-states/
For bulk purchases:
- Buy PLGA Polymer
https://resolvemass.ca/buy-plga-polymer/
Related Resources (Supplier & Manufacturing)
- Custom PLGA Synthesis Supplier: https://resolvemass.ca/custom-plga-synthesis-supplier/
- PLGA Contract Manufacturing: https://resolvemass.ca/plga-contract-manufacturing/
- PLGA Scale-Up Case Study: https://resolvemass.ca/plga-scale-up-case-study/
6: CONTROL STRATEGIES TO MINIMIZE SOLVENTS IN PLGA PRODUCTION
Upfront answer: Proper purification, solvent exchange, and vacuum drying drastically reduce PLGA Residual Solvents.
Key engineering controls
- Multi-stage precipitation
- Controlled vacuum drying
- Solvent exchange purification
- Nitrogen sweep drying
- Online solvent monitoring
Process case study:
- PLGA Microsphere Case Study
https://resolvemass.ca/plga-microsphere-case-study/
7: IMPACT OF RESIDUAL SOLVENTS ON PLGA DRUG RELEASE
Upfront answer: PLGA Residual Solvents influence drug release rate, microsphere porosity, Tg, and molecular weight retention.
Impact observations
- Elevated DCM → higher burst release
- Elevated acetone → weak microsphere structure
- Elevated Class 2 solvents → toxicological concern
Useful studies:
- PLGA Oncology Formulation
https://resolvemass.ca/plga-oncology-formulation/ - PLGA for Depot Formulation
https://resolvemass.ca/plga-depot-formulation/
8: DOCUMENTATION REQUIRED FOR ANDA/NDA SUBMISSIONS
Upfront answer: Regulatory dossiers require full solvent profiles, ICH Q3C compliance confirmation, and validated analytical methods.
Required documentation
- CoA solvent levels
- ICH Q3C compliance statement
- Validation package (ICH Q2)
- Stability studies
- Polymer equivalence reports
Helpful formulation and regulatory resources:
- PLGA Characterization for RLD
https://resolvemass.ca/plga-characterization-for-rld/ - Q1 Q2 Polymer Equivalence
https://resolvemass.ca/q1-q2-polymer-equivalence-assessment/
9: CASE STUDIES WHERE RESIDUAL SOLVENTS CAUSED FAILURE
Examples include:
- High DCM caused unexpected burst release in PLGA 50:50 microspheres
- ANDA rejection due to acetonitrile above allowable limits
- Nanoparticles becoming unstable from trapped IPA
Related technical case studies:
- PLGA Microencapsulation Scale-Up
https://resolvemass.ca/plga-microencapsulation-scale-up/ - Custom PLGA Release Control
https://resolvemass.ca/custom-plga-release-control/
CONCLUSION
PLGA Residual Solvents are central to determining the regulatory acceptance, safety, and performance of PLGA-based drug delivery systems. By ensuring strict ICH Q3C compliance, rigorous analytical testing, and supplier qualification, formulation scientists can guarantee polymer consistency and predictable drug release. ResolveMass Laboratories Inc. provides GMP-ready, low-solvent PLGA, comprehensive characterization, and regulatory support to ensure successful development of long-acting injectables, nanoparticles, depot systems, and oncology formulations.
FAQs ON PLGA RESIDUAL SOLVENTS
Residual solvents in PLGA are trace organic chemicals that remain after polymer synthesis, purification, or drying. They matter because even ppm-level quantities can affect polymer purity, toxicity, degradation kinetics, and drug–polymer interactions.
For injectable-grade microspheres, implants, and nanoparticles, excess solvents like dichloromethane (DCM) or tetrahydrofuran (THF) can affect:
-Drug stability during encapsulation
-Polymer molecular weight retention
-Release-rate uniformity
-Biocompatibility and toxicology outcomes
-Regulatory acceptance under ICH Q3C
Since PLGA is widely used in long-acting injectables and parenteral systems, controlling residual solvents is a mandatory requirement for safety and batch reproducibility.
The most common residual solvents in PLGA include dichloromethane (DCM), ethyl acetate, acetone, methanol, and tetrahydrofuran (THF). These are used during polymerization or purification steps for their ability to dissolve PLGA efficiently.
-DCM is frequently used because it dissolves both lactide and glycolide components effectively.
-Ethyl acetate and acetone are used during washing and precipitation.
-THF appears when certain ring-opening polymerization conditions are used.
-Methanol is sometimes used in final purification or washing stages.
Even though these solvents are part of many industrial processes, the final pharmaceutical-grade PLGA must meet strict ICH Q3C solvent limits.
Residual solvents in PLGA are typically evaluated using USP and ICH Q3C-aligned methodologies, most commonly:
-Headspace Gas Chromatography (HS-GC)
-GC-FID for quantification
-GC-MS for confirmation and trace-level identification
ICH Q2(R2) requires validation for: accuracy, precision, specificity, limit of detection (LOD), limit of quantification (LOQ), linearity, and robustness.
A fully validated method ensures that PLGA used for injectables meets global regulatory expectations for parenteral safety, toxicology, and quality consistency.
Acceptable limits depend on solvent classification:
-Class 1 solvents (e.g., benzene) — prohibited; must not be present.
-Class 2 solvents (e.g., DCM, THF, methanol) — strict limits based on toxicity (e.g., DCM 600 ppm, THF 720 ppm, methanol 3000 ppm).
-Class 3 solvents (e.g., acetone, ethyl acetate) — low, non-toxic solvents with limits around 5000 ppm.
Pharmaceutical-grade PLGA typically maintains residual solvents at far lower levels than these maximum allowable limits, and reputable suppliers publish solvent-level specifications on every Certificate of Analysis (COA).
Residual solvent levels are significantly influenced by:
-Type of polymerization (ROP vs. polycondensation)
-Solvent removal efficiency
-Vacuum drying cycles and temperature control
-Extent of solvent recovery and purification
-Polymer molecular weight (higher Mw retains solvents longer)
-Scale of production (large reactors require extended drying)
Suppliers with controlled synthesis, advanced purification, and validated drying conditions consistently achieve lower residual solvent profiles.
Reference
- ICH Guideline Q3C (R8): Impurities — Guideline for Residual Solvents.
International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH).https://www.ich.org - ICH Guideline Q2 (R2): Validation of Analytical Procedures.International Council for Harmonisation.https://www.ich.org/page/quality-guidelines
- Danhier, F., Ansorena, E., Silva, J.M., Coco, R., Le Breton, A., Préat, V. “PLGA-based nanoparticles: An overview of biomedical applications.”Journal of Controlled Release, 161(2), 505–522 (2012).https://doi.org/10.1016/j.jconrel.2012.01.003
- Fredenberg, S., Wahlgren, M., Reslow, M., Axelsson, A. “The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems.”International Journal of Pharmaceutics, 415(1–2), 34–52 (2011).https://doi.org/10.1016/j.ijpharm.2011.03.049

