OVERVIEW
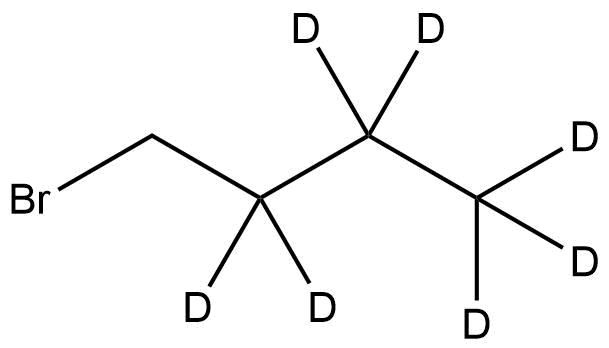
1-Bromobutane-2,2,3,3,4,4,4-d₇ is a deuterium-labeled alkyl halide consisting of a four-carbon chain with seven deuterium atoms substituted for hydrogen. This isotopically enriched analogue of 1-bromobutane is an essential reagent in analytical chemistry, isotope-dilution mass spectrometry, and synthetic organic research. The complete deuteration of the carbon skeleton significantly increases the molecular mass while maintaining the same chemical and physical behavior as the non-deuterated compound.
This compound is particularly useful as a labeled internal standard in chromatographic and spectroscopic studies, where its +7 Da mass difference enables precise quantification without interference. The bromine atom provides a reactive site for nucleophilic substitution and organometallic transformations, making it a key intermediate for the synthesis of deuterated alcohols, amines, esters, and other organic molecules.
At ResolveMass Laboratories Inc., 1-Bromobutane-2,2,3,3,4,4,4-d₇ is synthesized with exceptional isotopic enrichment and purity, ensuring accuracy and reproducibility for both analytical and research-grade applications.
CHEMICAL INFORMATION
-
Name: 1-Bromobutane-2,2,3,3,4,4,4-d7
-
Molecular Formula: C₄D₇Br
-
Molecular Weight: 144.06 g/mol
-
CAS Number: 223487-53-4
With its short carbon chain and extensive deuteration, this compound provides well-resolved isotopic patterns, making it suitable for precise isotopic ratio measurements and internal standardization in analytical methodologies.
APPLICATIONS
1. Internal Standard for GC-MS and LC-MS Quantification:
1-Bromobutane-d₇ serves as a robust internal standard in gas and liquid chromatography coupled with mass spectrometry. Its distinctive +7 Da mass shift relative to the unlabeled analogue allows accurate quantification of brominated hydrocarbons, ensuring analytical precision across varying sample matrices.
2. Reference Material in NMR Spectroscopy:
The absence of hydrogen-derived signals enhances spectral clarity in ^1H and ^13C NMR spectroscopy. The compound’s deuterated backbone provides a clean spectral baseline, making it ideal for chemical shift calibration, spectral interpretation, and solvent suppression studies.
3. Synthetic Intermediate for Deuterated Compounds:
Due to the reactivity of the bromine substituent, 1-Bromobutane-2,2,3,3,4,4,4-d₇ can undergo various nucleophilic substitution and metal-catalyzed reactions to yield deuterated derivatives such as n-butanol-d₇, butylamine-d₇, and deuterated surfactants. These compounds are important in pharmaceutical metabolism studies, tracer experiments, and labeled reagent synthesis.
4. Environmental Tracing and Hydrocarbon Fate Studies:
Its stability and isotopic label make it an excellent tracer in environmental systems for studying the behavior, volatility, and degradation of alkyl halides. Deuterium labeling allows precise mass spectrometric tracking during biodegradation or atmospheric transformation studies.
5. Investigation of Kinetic Isotope Effects (KIE):
1-Bromobutane-d₇ is used to explore kinetic isotope effects in substitution and elimination reactions. The replacement of C–H bonds with stronger C–D bonds enables researchers to study reaction rate variations and transition-state energetics in mechanistic organic chemistry.
6. Use in Materials and Surface Studies:
Its compact deuterated structure makes it useful for surface adsorption and diffusion studies, particularly in neutron reflectometry and thin-film investigations where isotopic contrast enhances structural resolution.
ADVANTAGES
-
High Deuterium Enrichment (≥98 atom % D): Ensures accurate isotopic labeling and reproducible analytical results.
-
Mass Shift (+7 Da): Enables easy identification and quantitation in mass spectrometric analyses.
-
Stable and Chemically Equivalent: Retains identical reactivity to non-deuterated 1-bromobutane.
-
Reduced Proton Interference: Enhances clarity in NMR and IR spectral studies.
-
Versatile Reactivity: Ideal for synthetic transformations involving nucleophilic substitution or alkylation.
-
Broad Research Utility: Applicable in analytical chemistry, isotope tracing, and mechanistic investigations.
HANDLING
-
Perform all manipulations in a fume hood or well-ventilated workspace.
-
Avoid inhalation, ingestion, or skin contact; use protective gloves, goggles, and lab coat.
-
Keep away from ignition sources and strong oxidizing agents.
-
Use only with appropriate inert materials and tools.
QUALITY & ANALYTICAL CHARACTERIZATION
Each batch of 1-Bromobutane-2,2,3,3,4,4,4-d₇ from ResolveMass Laboratories Inc. is synthesized and validated under strict quality control to ensure isotopic consistency and chemical integrity.
Analytical Verification Includes:
-
Mass Spectrometry (MS): Confirms molecular ion and isotopic composition.
-
NMR Spectroscopy (^1H, ^2H, ^13C): Verifies deuteration level and molecular structure.
-
Infrared Spectroscopy (IR): Detects characteristic C–D stretching vibrations.
-
Gas Chromatography (GC): Evaluates purity and absence of impurities.
All analytical data, including Certificate of Analysis (COA) and Safety Data Sheet (SDS), are provided to ensure traceability, reproducibility, and compliance with research standards.
SUMMARY
1-Bromobutane-2,2,3,3,4,4,4-d₇ (CAS 223487-53-4) is a high-purity, isotopically enriched alkyl bromide offering superior performance for analytical, synthetic, and isotopic research. Its stable deuterated structure, high enrichment, and predictable chemical reactivity make it indispensable in quantitative GC-MS calibration, isotope-tracing, and kinetic studies.
ResolveMass Laboratories Inc. guarantees consistent isotopic quality, precise analytical validation, and reliable supply — supporting researchers in achieving high-accuracy results in deuterium-based analytical and synthetic chemistry applications.
Learn more about,
Deuterated Polymers: A Cornerstone Guide to Synthesis, Applications, and Future Trends
Availability of All the Deuterated Chemicals at ResolveMass Laboratories Inc.
ResolveMass Laboratories: Leading Deuterated Chemical Synthesis Company in the United States.
Deuterated Internal Standards for LC-MS: Selection & Custom Synthesis
How to Choose the Right Deuterated Labelled Chemical Synthesis Company in Canada
How to Choose the Right Deuterium Labelled Compounds Supplier for Your Lab
Deuterium-Labelled Compounds — Synthesis, Applications & Ordering

Reviews
There are no reviews yet.