OVERVIEW
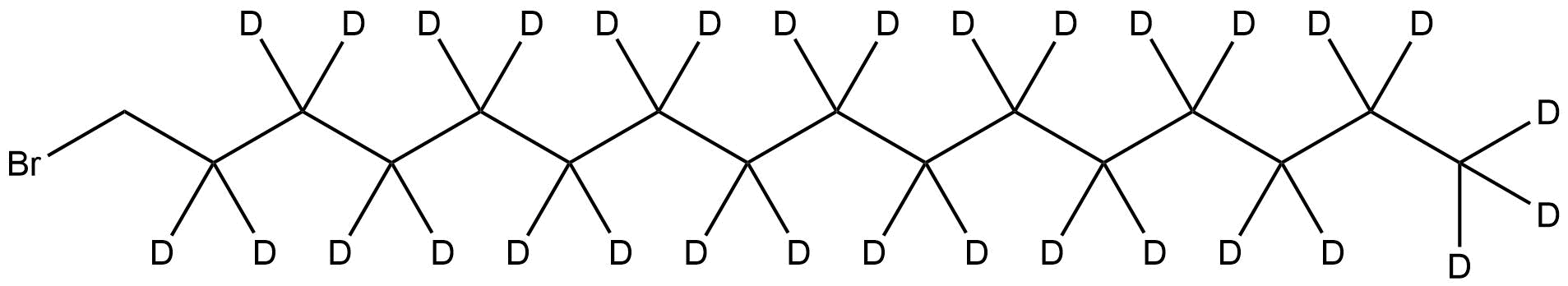
1-Bromohexadecane-2,2,3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,11,12,12,13,13,14,14,15,15,16,16,16-d₃₁ is a fully deuterated long-chain alkyl bromide that serves as a key compound in isotopic, spectroscopic, and surface chemistry studies. With thirty-one deuterium atoms incorporated along the hydrocarbon chain, this compound exhibits a substantial mass shift while preserving the structural and chemical properties of the non-deuterated analogue, 1-bromohexadecane.
This compound’s long hydrophobic chain and terminal bromine functionality make it an excellent intermediate for synthesizing deuterated surfactants, lipids, and organosilane precursors. The high level of deuterium enrichment enhances its value in neutron scattering experiments, tracer studies, and investigations of molecular dynamics in biological and polymeric systems.
Produced with exceptional isotopic precision and purity by ResolveMass Laboratories Inc., 1-Bromohexadecane-d₃₁ supports advanced research in materials science, biophysics, and analytical chemistry, offering a stable, reproducible, and isotopically well-defined reagent for diverse laboratory applications.
CHEMICAL INFORMATION
-
Name: 1-Bromohexadecane-2,2,3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,11,12,12,13,13,14,14,15,15,16,16,16–d31
-
Molecular Formula: C₁₆D₃₁Br
-
Molecular Weight: 336.53 g/mol
-
Isotopic Enrichment: ≥ 98 atom % D
-
Solubility: Insoluble in water; soluble in chloroform, ether, benzene, and other nonpolar organic solvents
The molecule consists of a fully deuterated C₁₆ alkyl chain terminated with a bromine atom at the 1-position. The substitution of deuterium for hydrogen reduces vibrational frequencies and enhances thermal and oxidative stability, making this compound particularly valuable for studying isotopic effects in organic and material systems.
APPLICATIONS Of 1-Bromohexadecane-2,2,3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,11,12,12,13,13,14,14,15,15,16,16,16–d31
1. Isotopic Tracer in Analytical and Environmental Studies:
1-Bromohexadecane-d₃₁ serves as an isotopic tracer in the analysis of long-chain brominated hydrocarbons, aiding in environmental monitoring and degradation studies. Its +31 Da mass difference from the non-deuterated analogue allows clear distinction in mass spectrometric detection, even in complex matrices such as sediments, biota, or atmospheric samples.
2. Precursor in the Synthesis of Deuterated Surfactants and Lipids:
The bromine functionality enables nucleophilic substitution reactions, allowing conversion to deuterated amines, alcohols, and quaternary ammonium salts. These derivatives are critical in producing deuterated surfactants and phospholipids for studying micelle formation, membrane interactions, and self-assembly in deuterated solvents or heavy water environments.
3. Material Science and Surface Chemistry:
In materials research, 1-Bromohexadecane-d₃₁ is employed as a reagent for surface modification and monolayer assembly on metal or silicon substrates. Its long-chain structure facilitates the formation of well-ordered self-assembled monolayers (SAMs), while deuteration enhances the visibility of surface structures in neutron reflectivity and vibrational spectroscopy.
4. Spectroscopic and Neutron Scattering Studies:
The high deuterium content makes this compound ideal for neutron scattering, vibrational (IR and Raman), and NMR experiments. Deuterium substitution minimizes proton background and provides clear isotopic contrast, aiding in the study of molecular dynamics, chain conformations, and vibrational coupling in complex systems.
5. Kinetic Isotope Effect (KIE) and Reaction Mechanism Studies:
Deuterium’s stronger C–D bond and lower zero-point energy lead to measurable kinetic isotope effects. This property enables researchers to use 1-Bromohexadecane-d₃₁ for probing reaction mechanisms, particularly in halogen-exchange reactions, substitution processes, and radical-mediated transformations involving long-chain bromides.
6. Stable Reference Standard for Mass Spectrometry and Chromatography:
Due to its predictable isotopic distribution and stability, 1-Bromohexadecane-d₃₁ is used as a calibration standard or reference material in GC-MS and LC-MS for quantifying long-chain brominated or alkyl compounds. The compound’s chromatographic behavior closely mimics its hydrogen analogue, ensuring precise analytical comparison.
ADVANTAGES of 1-Bromohexadecane-2,2,3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,11,12,12,13,13,14,14,15,15,16,16,16–d31
-
High Isotopic Enrichment (≥98 atom % D): Ensures analytical precision and reliable isotopic contrast.
-
Excellent Chemical Stability: Resistant to oxidation and degradation under standard conditions.
-
Long-Chain Structure: Facilitates ordered assembly in films and membranes.
-
Strong Mass Shift (+31 Da): Enables clear mass spectrometric differentiation.
-
Deuterium-Induced Spectroscopic Benefits: Reduced proton interference for NMR and IR studies.
-
Synthetic Versatility: Functional bromine site allows straightforward chemical modification.
HANDLING
1-Bromohexadecane-d₃₁ should be handled with appropriate laboratory precautions, as it is an alkyl halide capable of causing irritation and may release corrosive vapors under heat.
-
Conduct all operations in a well-ventilated fume hood.
-
Avoid contact with skin and eyes.
-
Use gloves, goggles, and protective clothing.
-
Avoid prolonged exposure to heat or open flames.
QUALITY & DOCUMENTATION
Every batch of 1-Bromohexadecane-d₃₁ produced by ResolveMass Laboratories Inc. undergoes stringent analytical verification to ensure isotopic enrichment, purity, and molecular integrity. Analytical testing typically includes:
-
Mass Spectrometry (MS): Confirms isotopic distribution and molecular mass.
-
NMR Spectroscopy (^1H, ^2H, ^13C): Verifies structural integrity and deuterium incorporation.
-
Infrared (IR) Spectroscopy: Identifies characteristic C–D and C–Br vibrations.
-
GC Purity Analysis: Ensures chemical homogeneity and absence of side-chain impurities.
All products are supplied with a Certificate of Analysis (COA), Safety Data Sheet (SDS), and traceability documentation, ensuring full transparency and reproducibility for research and industrial applications.
SUMMARY
1-Bromohexadecane-2,2,3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,11,12,12,13,13,14,14,15,15,16,16,16-d₃₁ is a highly deuterated long-chain alkyl bromide designed for isotopic research, surface science, and analytical chemistry. With its superior isotopic purity, structural stability, and versatile reactivity, it is indispensable in studies of molecular dynamics, surface assembly, and isotopic labelling.
ResolveMass Laboratories Inc. provides this compound with uncompromising quality and analytical consistency, ensuring researchers can rely on it for precision, reproducibility, and performance across diverse scientific disciplines.
Learn more about,
Deuterated Polymers: A Cornerstone Guide to Synthesis, Applications, and Future Trends
Availability of All the Deuterated Chemicals at ResolveMass Laboratories Inc.
ResolveMass Laboratories: Leading Deuterated Chemical Synthesis Company in the United States.
Deuterated Internal Standards for LC-MS: Selection & Custom Synthesis
How to Choose the Right Deuterated Labelled Chemical Synthesis Company in Canada
How to Choose the Right Deuterium Labelled Compounds Supplier for Your Lab
Deuterium-Labelled Compounds — Synthesis, Applications & Ordering
Reviews
There are no reviews yet.