1-CHLORO-2-NITRONAPHTHALENE | CAS 607-22-7
OVERVIEW
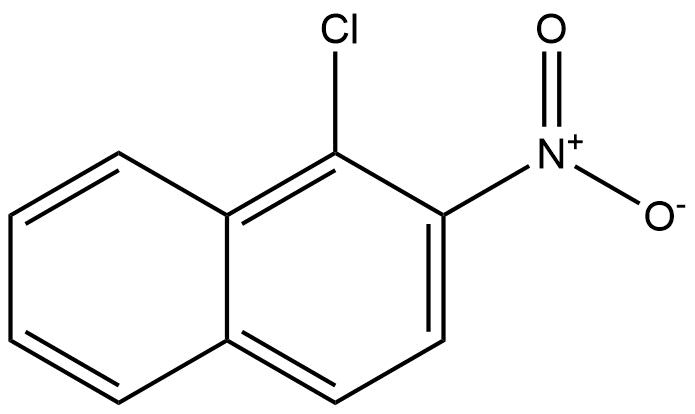
1-chloro-2-nitronaphthalene | CAS 607-22-7 is a halogenated nitroaromatic derivative of naphthalene that combines both chloro and nitro functional groups on a fused bicyclic aromatic ring system. This compound is an important intermediate in organic synthesis, especially in the manufacture of dyes, pharmaceuticals, agrochemicals, and specialty polymers. It is valued for its reactivity in electrophilic aromatic substitution and as a building block for more complex heterocyclic compounds.
Chemically identified as 1-chloro-2-nitronaphthalene, the molecule exhibits the following formula and identifiers:
-
CAS Number: 607-22-7
-
Molecular Formula: C₁₀H₆ClNO₂
-
Molecular Weight: 207.61 g/mol
-
Structure: Naphthalene core with a chlorine substituent at the 1-position and a nitro substituent at the 2-position
This unique substitution pattern confers distinct physicochemical and reactivity profiles, making it a versatile intermediate in industrial and research applications.
CHEMICAL & PHYSICAL PROPERTIES
1-Chloro-2-nitronaphthalene is a solid aromatic compound. Its salient properties are:
-
Appearance: Pale yellow to light tan crystalline solid
-
Odor: Mild aromatic/nitro character
-
Boiling/Melting Properties: Defined by firm crystalline lattice, tends to have a high melting point due to aromatic stacking
Due to its chlorinated and nitro functionalities, the compound displays high electron deficiency, enabling participation in various nucleophilic aromatic substitution and reduction reactions.
SYNTHESIS & CHEMICAL REACTIONS
Industrial and laboratory synthesis of 1-chloro-2-nitronaphthalene typically starts from naphthalene via stepwise electrophilic aromatic substitution:
-
Chlorination:
Naphthalene is first chlorinated under controlled conditions to yield 1-chloronaphthalene as the predominant isomer. This step often uses chlorine gas with a Lewis acid catalyst (e.g., FeCl₃). -
Nitration:
The 1-chloronaphthalene intermediate is then selectively nitrated using a nitrating mixture (e.g., nitric and sulfuric acids) to introduce the nitro group at the 2-position. Reaction conditions are optimized to minimize poly-substitution and rearrangement side products.
Because of the presence of both nitro and chloro groups, 1-chloro-2-nitronaphthalene participates in a variety of transformations:
-
Reduction of Nitro Group: Can be reduced to the corresponding amine (e.g., 1-chloro-2-aminonaphthalene), an important precursor for azo dye synthesis.
-
Substitution Reactions: The nitro group activates the aromatic ring towards nucleophilic substitution; the chloro group can be displaced under appropriate conditions.
-
Coupling Reactions: Under palladium or nickel catalysis, it can serve in cross-coupling reactions (e.g., Suzuki, Buchwald–Hartwig) to build larger aromatic frameworks.
APPLICATIONS of 1-chloro-2-nitronaphthalene | CAS 607-22-7
1-Chloro-2-nitronaphthalene functions predominantly as a synthetic intermediate across chemical industries:
1. Dye and Pigment Production
The compound’s nitroaromatic core makes it a key precursor for azo dyes and pigments. After reduction and coupling, derivatives contribute to a wide range of colorants used in textiles, inks, and plastics.
2. Pharmaceutical Intermediate
It can be advanced to heterocyclic scaffolds relevant to drug development. Its functional handles (Cl and NO₂) provide orthogonal reactivity for constructing complex molecules.
3. Agrochemical Synthesis
Used in the production of herbicides and insecticides, particularly where naphthalene derivatives impart activity against pests or influence environmental stability.
4. Materials Science
Building blocks derived from 1-chloro-2-nitronaphthalene contribute to high-performance polymers, liquid crystals, and specialty coatings due to their aromatic rigidity and substituent tunability.
TECHNICAL SPECIFICATIONS & QUALITY
At ResolveMass Laboratories Inc., 1-chloro-2-nitronaphthalene is offered with stringent quality control and customizable grades to support:
-
Research & Development (R&D)
-
Pilot scale synthesis
-
Industrial production
Typical specification parameters include:
| Parameter | Value |
|---|---|
| Purity | ≥ 98% (custom grades available) |
| Appearance | Crystalline solid |
| Assay Method | GC/LC calibrated against standards |
| Packaging | Sealed, moisture-resistant containers |
| Storage | Cool, dry place; inert atmosphere recommended |
PACKAGING OPTIONS
ResolveMass offers flexible packaging tailored to customer needs:
-
Small units for laboratory research
-
Intermediate drums for scale-up work
-
Bulk packaging for OEM usage
(Excluding specific packaging details per user preference)
WHY CHOOSE RESOLVEMASS?
At ResolveMass Laboratories Inc., we provide:
-
Reliable, high-purity chemicals with fast fulfillment
-
Technical support from experienced chemists
-
Custom synthesis and contract manufacturing options
Our commitment to quality ensures that 1-chloro-2-nitronaphthalene meets the demands of both innovation and industrial application.
![3-bromo-1H-pyrazolo[3,4-d]pyrimidin-4-amine | CAS 83255-86-1](https://resolvemass.ca/wp-content/uploads/2026/01/3-bromo-1H-pyrazolo34-dpyrimidin-4-amineCAS-83255-86-1-300x300.png)
Reviews
There are no reviews yet.