OVERVIEW
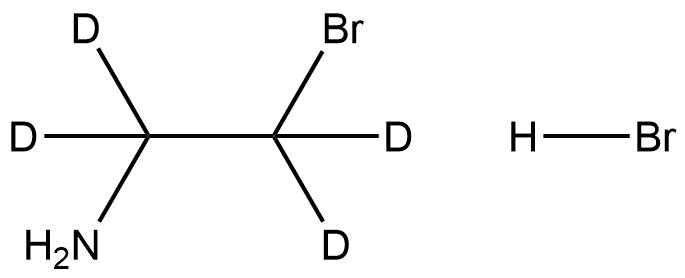
2-Bromoethyl-d₄-amine hydrobromide is a deuterium-labelled alkylamine salt that serves as a versatile intermediate and analytical reference compound. Structurally, it consists of a deuterated ethylamine backbone bearing a bromine substituent, with the compound stabilized in its hydrobromide salt form. The replacement of all four hydrogens on the ethyl chain with deuterium results in a +4 Da isotopic mass shift, enabling clear differentiation in analytical and mechanistic applications while maintaining the chemical behavior of the non-deuterated analogue.
This compound plays a key role in the synthesis of labelled biomolecules, pharmaceuticals, and radiotracers. The presence of both an amine and a bromine moiety allows it to function as a bifunctional reagent — participating in substitution, alkylation, and condensation reactions — while the hydrobromide salt enhances its stability and handling properties.
Produced under tightly controlled isotopic and chemical purity standards, 2-Bromoethyl-d₄-amine HBr from ResolveMass Laboratories Inc. provides high reliability for use in synthetic organic chemistry, isotope tracing, and spectroscopic analyses.
CHEMICAL INFORMATION
-
Name: 2-Bromoethyl-d₄-amine hydrobromide
-
Molecular Formula: C₂D₄Br₂N
-
Molecular Weight: 208.92 g/mol
-
CAS Number: 81764-55-8
-
Isotopic Enrichment: ≥ 98 atom % D
-
Stability: Stable under normal laboratory conditions; hygroscopic in nature
-
Functional Groups: Primary amine, alkyl bromide, and hydrobromide salt
Structurally, the compound features a bromine-substituted, fully deuterated ethylamine skeleton that enables facile participation in nucleophilic substitution and amine derivatization reactions. Its deuterium enrichment makes it particularly useful in metabolic and mechanistic isotope studies, where distinguishing labelled from non-labelled species is essential.
APPLICATIONS
1. Isotopic Labelling in Pharmaceutical and Metabolic Research:
2-Bromoethyl-d₄-amine HBr is widely used in the synthesis of deuterium-labelled drug intermediates and metabolites. The introduction of deuterium enhances the stability of C–D bonds, allowing researchers to investigate metabolic pathways, enzyme mechanisms, and isotope effects in vivo and in vitro.
2. Synthetic Intermediate for Aminoethyl Derivatives:
Due to the reactive bromine group, the compound serves as a key precursor for synthesizing a variety of deuterated amines, quaternary ammonium salts, and heterocyclic compounds. It readily undergoes substitution with nucleophiles such as thiols, amines, and alcohols to form deuterated analogues of bioactive molecules and surfactants.
3. Analytical Standard and Internal Reference in Mass Spectrometry:
The +4 Da isotopic mass shift of 2-Bromoethyl-d₄-amine HBr makes it an effective internal standard for quantitative GC-MS and LC-MS analyses of alkylamines and related compounds. Its identical chemical behavior to the non-deuterated analogue ensures accurate calibration and comparison during high-sensitivity measurements.
4. Research in Mechanistic and Kinetic Isotope Effects:
Deuterium substitution provides a slower rate of bond cleavage compared to protium, which is valuable for examining kinetic isotope effects in substitution, elimination, and amination reactions. This helps elucidate mechanistic pathways and transition states in organic synthesis and catalysis research.
5. Precursor for Radiochemical and Biochemical Labelling:
In combination with radioisotopic or fluorescent markers, 2-Bromoethyl-d₄-amine HBr is employed to produce labelled biomolecules for tracer studies. The compound’s amine functionality facilitates coupling to carboxyl or hydroxyl groups of biological molecules, while its deuterium content ensures analytical traceability in complex matrices.
6. Utility in Surface Chemistry and Polymer Modification:
The compound’s reactivity allows it to be grafted onto polymer backbones or surface films to introduce deuterated amine functionalities. This modification supports neutron scattering, infrared spectroscopy, and deuterium profiling of advanced materials.
ADVANTAGES
-
High Isotopic Purity (≥98 atom % D): Guarantees analytical reliability and isotopic precision.
-
Hydrobromide Salt Form: Enhances solid-state stability and ease of handling.
-
Dual Functional Reactivity: Contains both amine and alkyl bromide sites for versatile derivatization.
-
Chemically Equivalent to Non-Deuterated Analogue: Facilitates direct comparative studies in synthesis and analysis.
-
Mass Shift (+4 Da): Enables clear detection and quantification in isotope-dilution mass spectrometry.
-
Compatible with Aqueous and Organic Media: Ideal for both chemical and biochemical workflows.
HANDLING
2-Bromoethyl-d₄-amine HBr should be handled with appropriate care as it can release corrosive vapors and cause irritation upon contact. Laboratory personnel should follow standard safety procedures for amine and halogenated reagent handling.
-
Work in a fume hood or ventilated environment.
-
Avoid direct contact with skin and eyes.
-
Wear suitable protective gloves, safety glasses, and lab coats.
-
Prevent exposure to moisture, which may cause partial decomposition.
QUALITY & DOCUMENTATION
Each batch of 2-Bromoethyl-d₄-amine HBr manufactured by ResolveMass Laboratories Inc. is subject to rigorous analytical testing to ensure isotopic accuracy, chemical purity, and identity verification. Analytical methods include:
-
Mass Spectrometry (MS): Confirms molecular weight and deuterium incorporation.
-
NMR Spectroscopy (^1H, ^2H, ^13C): Verifies isotopic substitution and molecular structure.
-
Infrared (IR) Analysis: Detects characteristic amine and C–D vibrations, confirming compound integrity.
-
Elemental Analysis: Ensures conformity to expected stoichiometry and purity levels.
All products are supplied with a Certificate of Analysis (COA), Safety Data Sheet (SDS), and full batch traceability documentation, supporting compliance and reproducibility across research and industrial applications.
SUMMARY
2-Bromoethyl-d₄-amine hydrobromide (CAS 81764-55-8) is a high-purity, deuterium-labelled intermediate valued for its chemical versatility and isotopic precision. Combining the reactivity of a brominated amine with the stability of a hydrobromide salt, it serves as a reliable building block for pharmaceutical synthesis, isotope tracing, and mechanistic studies.
ResolveMass Laboratories Inc. ensures exceptional isotopic enrichment, verified quality, and reproducible performance — making 2-Bromoethyl-d₄-amine HBr a trusted choice for advanced research and analytical applications.
learn more about,
Deuterated Polymers: A Cornerstone Guide to Synthesis, Applications, and Future Trends
Reviews
There are no reviews yet.