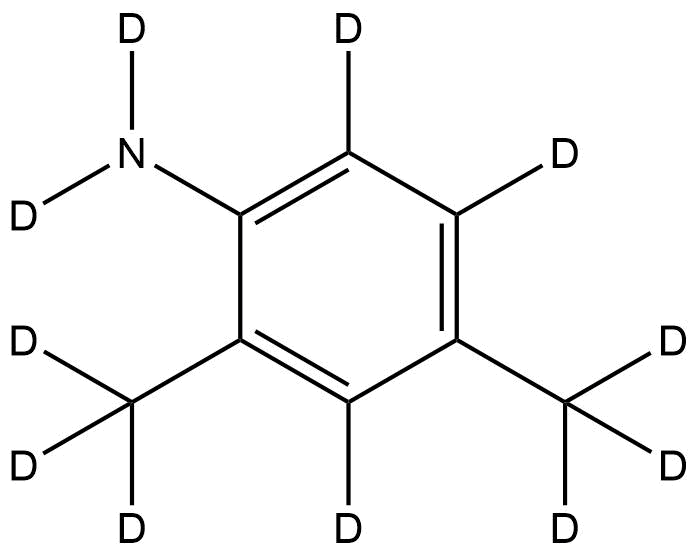
2,4-Dimethylaniline-d11 | CAS No. 1398065-83-2 | Isotopically Labeled Compound
Chemical Name: 2,4-Dimethylaniline-d11
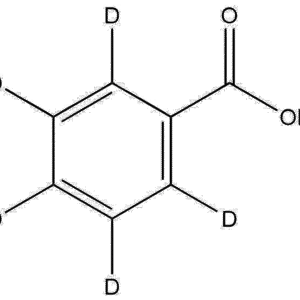
Molecular Formula: C8D11N
Molecular Weight: 132.16 g/mol
Synonyms: Deuterated 2,4-xylidine, 2,4-Xylidine-d11, 2,4-DMA-d11
Labeling: Deuterium (D, ²H) labeled analog of 2,4-Dimethylaniline
Isotopic Enrichment: ≥98 atom % D
Product Description
2,4-Dimethylaniline-d11 (CAS No. 1398065-83-2) is a fully deuterated analog of 2,4-Dimethylaniline, an aromatic amine widely used as an intermediate in the synthesis of pharmaceuticals, agrochemicals, and specialty dyes. In this isotopically labeled version, eleven hydrogen atoms in the molecular structure are replaced with deuterium atoms, offering enhanced utility in analytical and mechanistic studies requiring isotopic tracing and mass balance quantification.
The compound is typically supplied as a clear to pale yellow liquid or low-melting solid, depending on purity and storage conditions. Its deuterium labeling makes it especially valuable for stable isotope dilution mass spectrometry (SID-MS) and nuclear magnetic resonance (NMR) studies, where it serves as a precise internal standard for quantitative analyses and reaction tracking.
Structural and Isotopic Features
2,4-Dimethylaniline-d11 retains the core aromatic amine structure of its non-deuterated counterpart, featuring a benzene ring substituted with methyl groups at the 2- and 4-positions and an amino group at the 1-position. The replacement of hydrogen atoms with deuterium confers unique spectroscopic and kinetic properties, such as:
-
Increased molecular mass (+11 Da) due to deuterium substitution
-
Reduced proton NMR signal intensity, useful for background suppression in complex matrices
-
Altered vibrational frequencies in IR and Raman spectroscopy, facilitating isotopic differentiation
-
Enhanced stability against metabolic degradation in biological systems
These properties make 2,4-Dimethylaniline-d11 a powerful tracer molecule for isotope effect studies, kinetic isotope effect (KIE) measurements, and environmental fate research of aromatic amines.
Applications
1. Analytical Chemistry and Mass Spectrometry
Deuterated analogs such as 2,4-Dimethylaniline-d11 are indispensable in quantitative mass spectrometry (MS) and gas chromatography-mass spectrometry (GC-MS) methods. They serve as internal standards for accurate quantification of 2,4-Dimethylaniline and its derivatives in environmental samples, biological fluids, and industrial matrices. The presence of stable isotopic labeling ensures minimal co-elution interference and precise peak identification.
2. Pharmacokinetic and Metabolic Studies
In pharmaceutical R&D, 2,4-Dimethylaniline-d11 is utilized to study drug metabolism, bioaccumulation, and degradation pathways. Its deuterium labeling allows differentiation from endogenous or environmental analogs, enabling detailed tracking of metabolic transformations involving aromatic amines.
3. Mechanistic and Kinetic Studies
This compound is often employed in mechanistic organic chemistry to probe reaction kinetics and isotope effects, particularly in oxidation, nitration, and coupling reactions involving anilines. The substitution with deuterium can slow specific reaction pathways, helping chemists elucidate rate-determining steps and transition state characteristics.
4. Environmental and Toxicological Research
Due to the industrial relevance of 2,4-Dimethylaniline and related xylidines, the deuterated analog is used to trace pollutant degradation and transformation mechanisms in environmental systems. It assists in studying sorption, photolysis, and biodegradation kinetics in soil and aquatic media.
Technical Specifications
All batches are quality-tested using GC-MS, ¹H/²H NMR, and FTIR to confirm isotopic incorporation, chemical purity, and structural integrity.
Handling and Safety
2,4-Dimethylaniline-d11 should be handled in a well-ventilated fume hood, using appropriate personal protective equipment (gloves, goggles, and lab coat). Although the compound is isotopically labeled, it shares similar toxicological properties with 2,4-Dimethylaniline and should be treated with the same caution. Avoid skin or eye contact and inhalation of vapors.
For detailed hazard and safety information, refer to the Safety Data Sheet (SDS) available upon request.
Packaging and Availability
-
Pack Sizes: 100 mg, 500 mg, 1 g (custom pack sizes available upon request)
-
Lead Time: 2–4 weeks
-
Storage: Supplied in sealed amber glass bottles under inert gas to prevent oxidation and isotopic exchange.
Bulk quantities and custom isotopic enrichment levels can be provided for specialized research applications upon request.
Why Choose ResolveMass Laboratories Inc.?
At ResolveMass Laboratories Inc., we specialize in the synthesis, purification, and analytical characterization of isotopically labeled compounds. Our expertise in deuterium incorporation chemistry and high-resolution analytical validation (LC-MS, GC-MS, and NMR) ensures that every batch of 2,4-Dimethylaniline-d11 meets the highest standards of quality and isotopic integrity. We offer comprehensive analytical support, custom synthesis services, and global shipping to research organizations and pharmaceutical industries.
Deuterated Polymers: A Cornerstone Guide to Synthesis, Applications, and Future Trends
Reviews
There are no reviews yet.