OVERVIEW
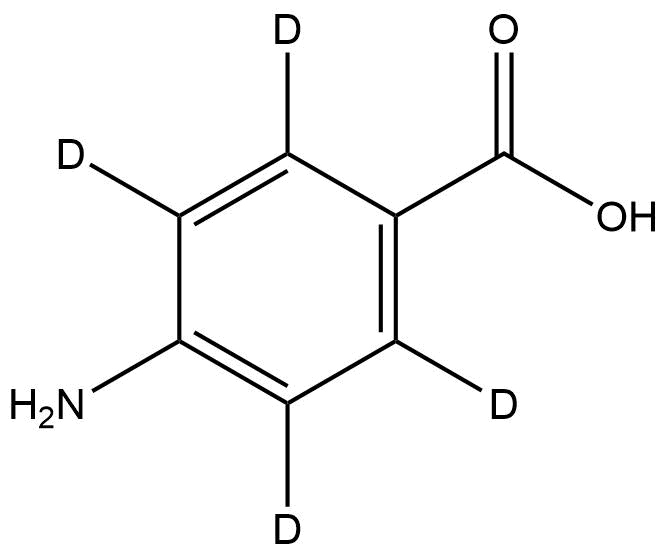
4-Aminobenzoic-2,3,5,6-d₄ Acid is a stable deuterium-labelled derivative of 4-aminobenzoic acid (commonly known as para-aminobenzoic acid or PABA). In this isotopologue, the four aromatic hydrogen atoms on the benzene ring are replaced by deuterium, resulting in a +4 Da increase in molecular weight compared to the non-labelled compound. Despite this isotopic substitution, the compound retains identical chemical structure, physical characteristics, and reactivity, making it an ideal internal standard and analytical reference.
4-Aminobenzoic-2,3,5,6-d₄ Acid is particularly valuable in analytical chemistry, metabolic research, and isotope-dilution mass spectrometry (IDMS). It serves as a reliable tracer and quantification standard in studies involving PABA metabolism, photochemistry, and vitamin-related biochemical pathways. The deuterium substitution enhances analytical precision without altering the compound’s fundamental biological or chemical behaviour.
CHEMICAL INFORMATION
-
Name: 4-Aminobenzoic-2,3,5,6-d₄ Acid
-
Molecular Formula: C₇H₃D₄NO₂
-
Molecular Weight: 141.16 g/mol
-
CAS Number: 350820-01-8
-
Isotopic Enrichment: Approximately 98–99 atom % D
-
Stability: Chemically and thermally stable under normal laboratory conditions; deuterium atoms remain intact under ambient storage
APPLICATIONS of 4-Aminobenzoic-2,3,5,6-d4 Acid | CAS 350820-01-8
-
Mass Spectrometry Internal Standard:
4-Aminobenzoic-2,3,5,6-d₄ Acid is widely used as an internal standard in LC-MS and GC-MS analyses of para-aminobenzoic acid and related compounds. The +4 Da mass shift allows precise differentiation between labelled and unlabelled species, ensuring high accuracy in quantitative determinations. -
NMR Spectroscopy:
The replacement of aromatic hydrogens with deuterium reduces proton signal interference, making it ideal for use in NMR studies involving aromatic compounds and for solvent suppression experiments. -
Metabolic Tracing and Biochemical Research:
As an isotopically labelled form of PABA—a compound known for its role in folic acid biosynthesis—this derivative is used in biochemical studies investigating metabolic pathways, enzymatic conversions, and nutrient dynamics. -
Analytical Method Development and Validation:
Employed as a reference compound in analytical method validation for pharmaceuticals, vitamins, and biological samples. Its isotopic label ensures consistency across repeated analyses and aids in calibration curve generation. -
Reaction Mechanism and Isotope Effect Studies:
The deuterium substitution at aromatic positions allows chemists to explore isotopic effects in reaction kinetics, hydrogen exchange processes, and aromatic substitution mechanisms.
ADVANTAGES of 4-Aminobenzoic-2,3,5,6-d4 Acid | CAS 350820-01-8
-
High isotopic enrichment (≥ 98 atom % D) ensures precise mass differentiation in isotope-based assays.
-
Retains identical chemical behaviour to native PABA, ensuring accurate comparative analysis.
-
Provides a consistent +4 Da isotopic mass shift, enabling unambiguous identification in mass spectrometry.
-
Enhanced spectral clarity in NMR due to reduced proton interference.
-
Highly stable under standard laboratory and storage conditions.
-
Suitable for use in multiple research fields, including biochemistry, analytical chemistry, and pharmacokinetics.
HANDLING
4-Aminobenzoic-2,3,5,6-d₄ Acid is considered a low-hazard compound when handled according to standard laboratory safety protocols.
-
Avoid inhalation of dust and direct contact with eyes or skin.
-
Use appropriate protective gear such as gloves, goggles, and a lab coat.
-
Handle in a well-ventilated area or fume hood.
-
Store in sealed containers, away from moisture, strong oxidisers, and direct sunlight.
-
Wash thoroughly after handling, and keep container tightly closed when not in use.
QUALITY & SPECIFICATION
Each batch of 4-Aminobenzoic-2,3,5,6-d₄ Acid is subjected to rigorous analytical testing to confirm isotopic enrichment, purity, and identity. Analytical characterization typically includes NMR spectroscopy, infrared spectroscopy, and mass spectrometry. The compound generally exhibits ≥ 98 % chemical purity and ≥ 99 % isotopic enrichment. Each lot is accompanied by a Certificate of Analysis (COA) confirming these specifications and ensuring full traceability for research and analytical use.
SUMMARY
4-Aminobenzoic-2,3,5,6-d₄ Acid (CAS 350820-01-8) is a high-quality, deuterium-labelled isotopologue of para-aminobenzoic acid (PABA). With its excellent isotopic enrichment and chemical stability, it is an ideal internal standard and tracer for analytical and biochemical studies. Its versatility across mass spectrometry, NMR spectroscopy, and metabolic research makes it an indispensable reagent in modern isotope chemistry and life sciences laboratories, supporting accurate quantitation, mechanistic studies, and isotopic tracing applications.
Learn more about,
Deuterated Polymers: A Cornerstone Guide to Synthesis, Applications, and Future Trends
Availability of All the Deuterated Chemicals at ResolveMass Laboratories Inc.
ResolveMass Laboratories: Leading Deuterated Chemical Synthesis Company in the United States.
Deuterated Internal Standards for LC-MS: Selection & Custom Synthesis
How to Choose the Right Deuterated Labelled Chemical Synthesis Company in Canada
How to Choose the Right Deuterium Labelled Compounds Supplier for Your Lab
Deuterium-Labelled Compounds — Synthesis, Applications & Ordering

Reviews
There are no reviews yet.