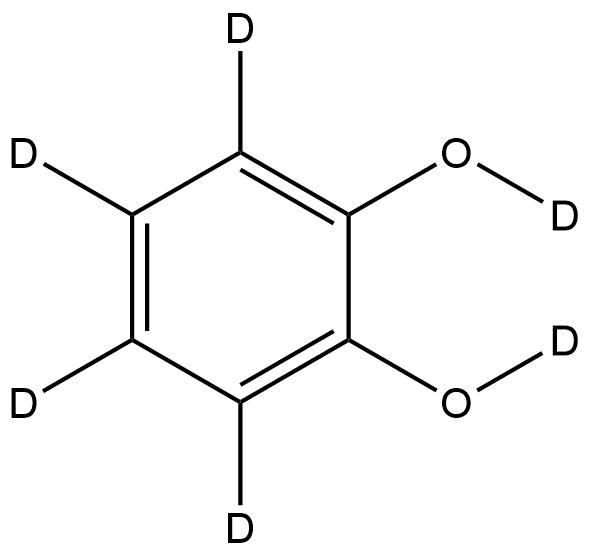
Catechol-d6 | CAS No.: 202656-22-2 | Fully Deuterated Phenolic Standard
Chemical Name: Catechol-d6
Molecular Formula: C6D6O2
Molecular Weight: 116.15 g/mol
Synonyms: 1,2-Benzenediol-d6, o-Dihydroxybenzene-d6, o-Hydroquinone-d6, Benzene-1,2-diol-d6
Isotopic Labeling: Fully deuterated (six deuterium atoms replacing aromatic hydrogens)
Isotopic Enrichment: ≥98 atom % D
Product Overview
Catechol-d6 (CAS No. 202656-22-2) is the fully deuterated isotopologue of catechol (1,2-benzenediol) — a dihydroxybenzene compound widely used in organic synthesis, biochemical studies, and environmental analysis. In this labeled analog, all six hydrogen atoms on the benzene ring are replaced with deuterium atoms, providing a stable, non-radioactive isotopic tracer ideal for quantitative and mechanistic studies.
Due to its identical chemical behavior and isotopic mass shift (+6 Da), Catechol-d6 serves as a reliable internal standard in mass spectrometry (MS) and nuclear magnetic resonance (NMR) spectroscopy. It is frequently used in analytical assays to quantify catechol, study oxidative degradation pathways, or evaluate enzymatic reactions involving catechol derivatives such as dopamine, epinephrine, and phenolic antioxidants.
Structural and Isotopic Features
Catechol (1,2-dihydroxybenzene) features two hydroxyl groups at adjacent positions (ortho configuration) on an aromatic ring. In Catechol-d6, each aromatic hydrogen is replaced with a deuterium atom, resulting in a heavier, isotopically distinct molecule without altering its electronic or structural properties.
This isotopic substitution offers several analytical and kinetic advantages:
-
Stable isotope differentiation: +6 Da mass shift relative to catechol
-
Identical chromatographic retention to native catechol
-
Distinct m/z profile in LC-MS/MS or GC-MS for precise quantitation
-
Reduced background interference in complex biological or environmental matrices
-
Enhanced stability in isotopic tracing and degradation studies
Because of its non-radioactive and chemically inert nature, Catechol-d6 is an ideal standard for both routine analytical calibration and advanced kinetic isotope effect (KIE) studies.
Applications
1. Internal Standard for LC-MS and GC-MS Quantification
Catechol-d6 is widely used as a stable isotope-labeled internal standard in analytical chemistry. Its physicochemical properties mirror those of unlabeled catechol, ensuring identical chromatographic behavior, while the 6 Da mass difference allows unambiguous peak discrimination during mass spectrometric detection.
Typical applications include:
-
Quantitative determination of catechol and related dihydroxybenzenes in biological fluids (urine, plasma, cerebrospinal fluid)
-
Monitoring phenolic intermediates in oxidative degradation pathways
-
Environmental trace analysis of phenolic pollutants
-
Validation of isotope dilution mass spectrometry (IDMS) methods
Using Catechol-d6 as an internal reference corrects for sample loss, ion suppression, and extraction variability, enabling highly accurate and reproducible quantification.
2. Mechanistic and Kinetic Isotope Effect Studies
In mechanistic organic chemistry, Catechol-d6 serves as a powerful tool for investigating reaction kinetics and isotopic substitution effects.
The stronger carbon–deuterium (C–D) bonds compared to C–H bonds exhibit lower vibrational energy, slowing reaction rates in processes involving C–H cleavage. Researchers use Catechol-d6 to study:
-
Oxidation and redox kinetics of catechols and quinones
-
Enzymatic oxidation by tyrosinase, peroxidase, or polyphenol oxidase
-
Radical and electron-transfer mechanisms
-
Photodegradation and polymerization reactions
By comparing the reactivity of labeled versus unlabeled catechol, scientists can infer the rate-determining steps and mechanistic pathways of oxidative transformations.
3. NMR Spectroscopy and Instrument Calibration
Catechol-d6 provides distinct advantages in NMR spectroscopy as a reference or solvent standard for both ¹H and ²H NMR systems.
In ¹H NMR, the absence of proton resonances simplifies spectra, facilitating interpretation in proton-rich systems. In ²H NMR, Catechol-d6 provides a well-defined deuterium resonance used for field-frequency locking and quantitative deuterium analysis.
The compound’s thermal and chemical stability make it ideal for long-term instrument calibration and NMR system validation.
4. Environmental and Industrial Research
Catechol and its derivatives are key intermediates in industrial synthesis, polymer production, dye manufacture, and antioxidant formulations. Catechol-d6 enables isotopic tracing of degradation, transformation, and migration of phenolic compounds in industrial and environmental systems.
Researchers use it to study:
-
Microbial degradation pathways of aromatic pollutants
-
Biotransformation kinetics in soil and water
-
Oxidative stability of phenolic-based materials
Such studies are critical in environmental toxicology and green chemistry for assessing pollutant behavior and fate.
Technical Specifications
Each batch of Catechol-d6 undergoes comprehensive quality control using LC-MS, ¹H/²H NMR, and FTIR spectroscopy to verify isotopic incorporation, purity, and structural integrity.
Handling and Safety
Catechol-d6 is chemically stable and non-radioactive but should be handled with care similar to other phenolic compounds.
Avoid inhalation or direct skin contact; use gloves, protective eyewear, and lab coats during handling. Perform all procedures in a well-ventilated area or fume hood.
Refer to the Safety Data Sheet (SDS) for complete hazard, handling, and disposal information.
Why Choose ResolveMass Laboratories Inc.?
At ResolveMass Laboratories Inc., we specialize in the custom synthesis and analytical characterization of stable isotope-labeled compounds for the pharmaceutical, environmental, and chemical research sectors.
Our ISO 9001:2015-certified facility ensures rigorous quality control, traceability, and analytical validation of every batch.
With expertise in deuterium incorporation chemistry, HPLC purification, and mass spectrometric verification, we deliver high-purity deuterated compounds like Catechol-d6 with consistent quality, reliability, and global delivery support.
Reviews
There are no reviews yet.