OVERVIEW
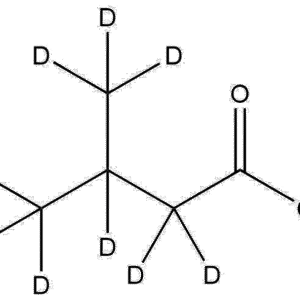
Deuterated 2-(Dimethylamino)ethyl methacrylate-d5 | CAS 2867-47-2, is a specialized isotope-labeled monomer widely used in polymer science, analytical chemistry, kinetic studies, and advanced material development. Incorporating five deuterium atoms into the dimethylamino functional group provides enhanced structural resolution in spectroscopic analysis while maintaining the chemical reactivity and polymerization behavior identical to its non-deuterated analogue.
At ResolveMass Laboratories Inc., DMAEMA-d5 is supplied as a high-purity monomer suitable for demanding R&D applications, including controlled radical polymerization (CRP), RAFT and ATRP polymer synthesis, isotopic tracing, and formulation mechanisms that require precise tracking of polymer behavior. The deuterated label enables clear differentiation in NMR, MS, and IR, making this monomer an excellent choice for mechanistic investigations and isotope-sensitive analytical workflows.
CHEMICAL INFORMATION
Chemical Name: Deuterated 2-(Dimethylamino)ethyl methacrylate-d5
CAS Number: 2867-47-2
Synonyms: DMAEMA-d5, Dimethylaminoethyl methacrylate-d5, Deuterated DMAEMA
Formula: C7H6D5NO2
Molecular Weight: 162.24 g/mol (isotope-adjusted)
Isotopic Labeling: Five deuterium atoms incorporated into the dimethylamino methyl groups
Functional Groups: Methacrylate ester + tertiary amine
The methacrylate moiety ensures efficient copolymerization with hydrophilic, hydrophobic, ionic, and zwitterionic monomers. Meanwhile, the tertiary amine contributes tunable cationic behavior, pH responsiveness, and electrostatic interaction capabilities. Deuteration does not alter the monomer’s polymerization kinetics in a significant way, ensuring compatibility with existing formulation protocols.
KEY FEATURES
-
High isotopic enrichment for precise analytical tracking
-
Preserved polymerization efficiency comparable to non-deuterated DMAEMA
-
Excellent compatibility with RAFT, ATRP, FRP, and other radical polymerization systems
-
Strong NMR visibility enabling accurate mechanistic and kinetic studies
-
Useful for mass spectrometry-based quantification and isotopic dilution analysis
-
Highly versatile in the development of advanced functional and stimuli-responsive polymers
APPLICATIONS of Deuterated 2-(Dimethylamino)ethyl methacrylate-d5 | CAS 2867-47-2
1. Polymer Synthesis & Mechanistic Studies
DMAEMA-d5 is extensively used in polymerization research—especially in ATRP, RAFT, and free radical polymerization—to track monomer conversion, determine chain-transfer events, and evaluate block copolymer formation. The deuterium label offers unmatched clarity in ^1H NMR due to suppressed proton signals, allowing researchers to characterize complex polymer architectures with higher confidence.
2. Stimuli-Responsive & Smart Materials
Polymers containing DMAEMA functionality exhibit pH-sensitive behavior, making them suitable for research in:
-
switchable hydrogels
-
pH-responsive drug delivery systems
-
biomaterials for gene or nucleic acid transport
-
self-assembling nanostructures
Incorporating a deuterated version facilitates deeper study of swelling behavior, phase transitions, and molecular mobility.
3. NMR & MS-Based Analytical Chemistry
The presence of deuterium atoms makes DMAEMA-d5 valuable for:
-
internal standards in NMR quantification
-
isotope ratio evaluation
-
mass spectrometric analysis
-
tracing degradation pathways
-
monitoring polymer–solvent interactions
Because deuterium provides spectroscopic contrast without chemically altering the monomer functionality, it is ideal for revealing subtle interactions during polymer formation and processing.
4. Surface Functionalization & Coating Research
DMAEMA-derived polymers are common in surface-modifying research for their ability to impart:
-
hydrophilicity
-
anti-fouling behavior
-
tunable charge
-
crosslinkable functionality
The deuterated variant enables researchers to characterize surface grafting efficiency via isotopically sensitive methods and differentiate grafted layers from underlying substrates.
5. Biomedical & Biotechnology Investigations
In early-stage biomedical research, DMAEMA-d5 can be employed to study:
-
polymer-cell interactions
-
gene transfection efficiencies
-
polymer degradation fingerprints
-
drug–polymer compatibility
Deuterium labeling is especially beneficial when tracking polymer uptake or biodegradation using LC–MS or NMR metabolomic workflows.
ADVANTAGES OF USING DMAEMA-D5 IN RESEARCH
-
Enhanced data clarity: Deuterium eliminates overlapping proton signals, improving spectral resolution.
-
Accurate mechanistic insights: Ideal for kinetic modeling and understanding reaction progression.
-
Compatibility with existing processes: No significant deviation in polymerization performance compared to non-deuterated DMAEMA.
-
Improved quantitative analysis: Easy to distinguish and quantify via MS or isotope-assisted NMR experiments.
-
Supports advanced materials innovation: Enables development of next-generation functional polymers with traceability.
STORAGE & HANDLING
DMAEMA-d5 should be stored under inert atmosphere conditions (such as nitrogen) and protected from light and heat to prevent premature polymerization. Refrigeration is recommended, and inhibitors typically present in methacrylate monomers should not be removed prior to use unless necessary for the intended polymerization process.
CONCLUSION
Deuterated 2-(Dimethylamino)ethyl methacrylate-d5 is an indispensable isotope-labeled monomer for modern polymer science, analytical characterization, and stimuli-responsive material research. Its precise labeling, strong analytical contrast, and robust polymerization performance make it a preferred choice for researchers working on next-generation materials, biomedical tools, and detailed mechanistic polymer studies. ResolveMass Laboratories Inc. is committed to delivering high-purity, research-grade DMAEMA-d5 to support scientific innovation across chemistry, biotechnology, and material science disciplines.
Learn more through,
Deuterated Polymers: A Cornerstone Guide to Synthesis, Applications, and Future Trends
Availability of All the Deuterated Chemicals at ResolveMass Laboratories Inc.
ResolveMass Laboratories: Leading Deuterated Chemical Synthesis Company in the United States.
Deuterated Internal Standards for LC-MS: Selection & Custom Synthesis
How to Choose the Right Deuterated Labelled Chemical Synthesis Company in Canada
How to Choose the Right Deuterium Labelled Compounds Supplier for Your Lab
Deuterium-Labelled Compounds — Synthesis, Applications & Ordering
Custom Synthesis of Deuterated Chemicals


Reviews
There are no reviews yet.