OVERVIEW
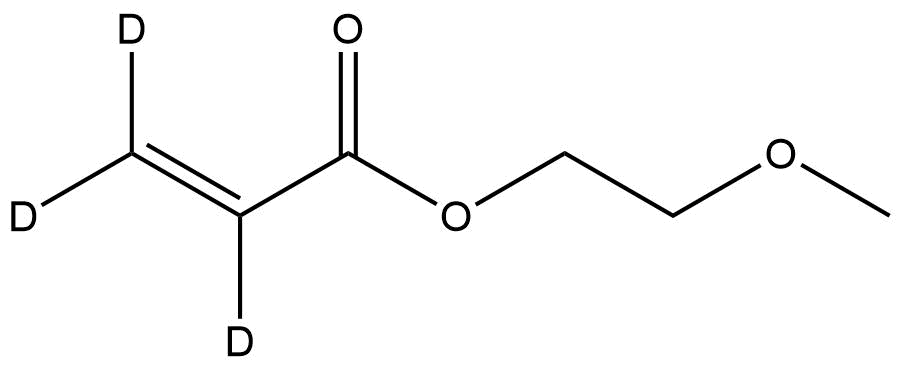
Deuterated 2-(Methoxy)ethyl acrylate-d3 is a highly specialized, isotopically enriched acrylate monomer widely used in polymer chemistry, materials science, reaction mechanism elucidation, and analytical method development. By replacing key hydrogen atoms with deuterium (²H), this monomer provides enhanced value for researchers who require precise kinetic tracing, mass-spectrometric discrimination, and improved stability in complex reaction matrices. Its structural similarity to the non-deuterated analogue ensures predictable polymerization behavior, while the isotopic labeling introduces measurable mass offsets essential for quantitative studies.
ResolveMass Laboratories Inc. supplies premium-grade deuterated monomers suited for advanced R&D, controlled polymerization studies, NMR tracing, and isotopic labeling applications across pharmaceutical, polymer, and chemical research environments. Deuterated 2-(Methoxy)ethyl acrylate-d₃ is synthesized with high isotopic enrichment to support reproducibility and analytical accuracy.
CHEMICAL INFORMATION
Chemical Name:
Deuterated 2-(Methoxy)ethyl acrylate-d₃
(Also referred to as 2-(Methoxy)ethyl acrylate-d₃ or MEA-d₃)
Synonyms:
• 1,1,2-d₃-2-(Methoxy)ethyl acrylate
• MEA-d₃
• Acrylate-d₃ derivative
• Deuterium-labeled 2-(2-methoxyethyl) acrylate
• D₃-MEA
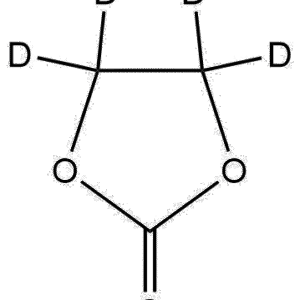
General Formula: C₆H₇D₃O₃
(Exact number of deuterium atoms may vary by supplier depending on labeling site; the d₃ variant typically features deuteration on the α-methylene region.)
Functional Groups:
• Acrylate ester
• Ether (methoxy group)
• Deuterium-labeled alkyl chain
Physical Form:
Typically a clear, colorless to slightly yellow liquid with acrylate-typical reactivity and viscosity properties.
This monomer is engineered to retain the chemical reactivity of standard 2-(methoxy)ethyl acrylate while offering a distinct isotopic signature useful for structural identification and quantification.
KEY FEATURES
• Isotopic enrichment (d₃) for MS-based differentiation
• Retention of standard acrylate polymerization kinetics
• High purity, low inhibitor content suitable for controlled polymerization work
• Compatible with RAFT, ATRP, and free-radical mechanisms
• Excellent solvency and miscibility with common organic solvents
• Stable isotopic label that remains intact under typical polymerization and analytical conditions
APPLICATIONS of Deuterated 2-(Methoxy)ethyl acrylate-d3
1. Polymer Chemistry & Controlled Radical Polymerization
Deuterated 2-(Methoxy)ethyl acrylate-d₃ is widely used in:
• Mechanistic studies of free-radical polymerization
• Determining monomer incorporation ratios
• Monitoring copolymerization kinetics
• Tracing polymer chain growth using NMR or MS fragmentation
The deuterium label allows researchers to track monomer units within complex polymer matrices, enabling high-fidelity chain-growth analysis and sequence distribution studies.
2. Mass Spectrometry (MS) and LC-MS Method Development
Deuterium labeling produces a predictable mass shift of +3 Da, making the monomer and its derivatives easily distinguishable from non-labeled species. This is valuable for:
• Internal standards for quantitative analysis
• Isotopic spike studies
• Fragmentation pathway analysis
• Polymer degradation tracking
3. Isotopic Dilution Analysis
Because deuterated compounds serve as ideal internal standards, MEA-d₃ is employed to improve quantitative accuracy in trace-level analysis of acrylate-based materials and their transformation products.
4. Reaction Mechanism & Kinetics Research
Deuterium kinetic isotope effects (KIE) help researchers evaluate:
• Rate-determining steps
• Radical reactivity differences
• Stability of acrylate intermediates
• Migration and side-reaction pathways
5. Advanced Materials & Surface Engineering
MEA-d₃ is incorporated in the synthesis of:
• Deuterated hydrogels
• Surface-functionalized polymer films
• Coatings requiring precise analytical validation
• Reference materials for polymer structural elucidation
TECHNICAL ADVANTAGES FOR RESEARCHERS
Enhanced Analytical Traceability
The deuterium (d₃) label allows clean separation and identification in mass spectra, reducing ambiguity in complex mixtures and supporting accurate quantification.
Consistent Polymerization Behavior
Because the chemical reactivity of acrylates is dominated by the vinyl group, the introduction of deuterium on the side chain does not significantly disrupt polymerization rates, ensuring predictable outcomes.
High Compatibility Across Analytical Platforms
MEA-d₃ performs exceptionally well in:
• HRMS
• GC-MS (with derivatization)
• LC-MS
• ¹H/²H NMR
• FTIR for isotopic shift studies
Improved Scientific Rigor
Using an isotopically labeled analogue ensures reproducibility, traceability, and regulatory-compliant documentation in advanced research.
STORAGE & HANDLING NOTES
• Store under dry, cool, inert atmosphere conditions.
• Protect from light and heat to prevent premature polymerization.
• Use with standard acrylate handling precautions—gloves, eye protection, and appropriate ventilation.
• Inhibitor may be present to improve shelf stability; remove via standard purification if required for sensitive polymerization experiments.
WHY CHOOSE RESOLVEMASS LABORATORIES INC.
ResolveMass Laboratories Inc. specializes in high-purity deuterated monomers and isotopically labeled intermediates tailored for pharmaceutical R&D, polymer science, and analytical method development. Our products emphasize reproducibility, technical documentation, and research-grade quality to support innovators in academia, biotechnology, and polymer/materials engineering.
Learn more through,
Deuterated Polymers: A Cornerstone Guide to Synthesis, Applications, and Future Trends
Availability of All the Deuterated Chemicals at ResolveMass Laboratories Inc.
ResolveMass Laboratories: Leading Deuterated Chemical Synthesis Company in the United States.
Deuterated Internal Standards for LC-MS: Selection & Custom Synthesis
How to Choose the Right Deuterated Labelled Chemical Synthesis Company in Canada
How to Choose the Right Deuterium Labelled Compounds Supplier for Your Lab
Deuterium-Labelled Compounds — Synthesis, Applications & Ordering
Custom Synthesis of Deuterated Chemicals
Reviews
There are no reviews yet.