OVERVIEW
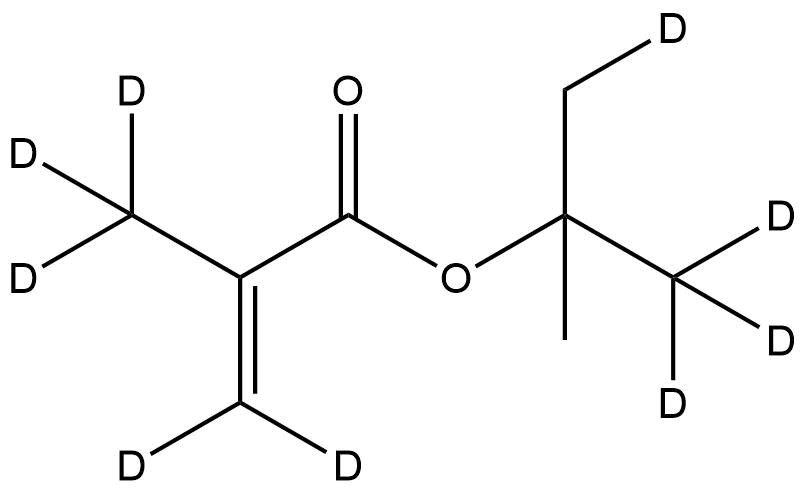
Deuterated tert-Butyl methacrylate-d9 is a stable-isotope-labeled monomer in which nine hydrogen atoms of the tert-butyl group are substituted with deuterium. This fully deuterated variant maintains the reactivity and steric profile of conventional tert-butyl methacrylate while providing exceptional isotopic contrast for analytical, mechanistic, and polymer science applications. Its unique isotopic composition makes it indispensable for advanced studies in polymerization kinetics, degradation tracing, nanoscale materials research, and quantitative NMR or MS-based investigations.
ResolveMass Laboratories Inc. supplies high-purity, research-grade Deuterated tert-Butyl methacrylate-d9 designed for synthetic chemistry, polymer engineering, and spectroscopic workflows where precision and reproducibility are non-negotiable. With stringent quality control and robust documentation, this monomer supports cutting-edge academic, industrial, and pharmaceutical R&D projects requiring isotopically enriched methacrylate systems.
CHEMICAL INFORMATION
Chemical Name: Deuterated tert-Butyl methacrylate-d9
Synonyms: tBMA-d9; tert-Butyl 2-methyl-2-propenoate-d9; t-Butyl methacrylate-d9
Formula: C8D9O2
Isotopic Enrichment: ≥98% D
Functional Group: Methacrylate ester
Physical Form: Colorless, volatile liquid
Reactivity: Supports free-radical, RAFT, and ATRP polymerization
The deuterated tert-butyl group provides a significantly shifted NMR signature, allowing researchers to track polymer end groups, branching, chain transfer, or degradation without spectral overlap from hydrogen atoms. Additionally, the deuterium mass shift of +9 amu delivers high-confidence detection in LC-MS and GC-MS, making it ideal for tracer studies or internal standards.
STRUCTURAL & ANALYTICAL ADVANTAGES
Deuterated tert-Butyl methacrylate-d9 is engineered to offer analytical clarity while retaining chemical compatibility with established methacrylate polymerization systems. Key analytical benefits include:
-
Enhanced NMR resolution: Deuterium substitution eliminates proton coupling patterns, simplifying ¹H and ¹³C NMR interpretation and enabling direct monitoring of polymer chain dynamics.
-
Mass spectrometric distinction: Its +9 amu isotopic offset ensures clear MS peak separation from non-deuterated analogs, improving quantification accuracy in complex mixtures.
-
Minimal kinetic isotope effect in polymerization: The monomer maintains reactivity similar to tBMA, enabling direct comparison between hydrogenated and deuterated polymer systems.
-
Reliable internal standard: Ideal for calibration curves and QC workflows in chemical, materials, and pharmaceutical laboratories.
APPLICATIONS of Deuterated tert-Butyl methacrylate-d9
Deuterated tert-Butyl methacrylate-d9 is widely used in specialty polymer synthesis, kinetic analysis, and spectroscopic research. Its applications include:
1. Polymerization Mechanism Studies
Researchers use tBMA-d9 to probe reaction pathways, chain propagation rates, and termination events. Deuterium labeling enables separation of isotopic fragments during MS and allows direct quantification of side-reactions such as chain transfer or decomposition.
2. Advanced Materials Development
Polymers derived from tBMA-d9 serve as analytical analogs for mapping diffusion, phase separation, or crosslink density in block copolymers, hydrophobic coatings, or thermo-responsive materials.
3. RAFT and ATRP Polymer Analysis
Isotopic labeling provides high-resolution tracking of living polymerization processes. Deuterated end-groups help confirm molecular weight distribution models, PDI behavior, and initiator-to-polymer fidelity.
4. Nuclear Magnetic Resonance Research
Deuterated monomers are essential in multi-dimensional NMR, contrast variation, and suppression experiments—particularly where proton-rich matrices obscure signals.
5. Mass Spectrometry Tracer Studies
In pharmaceutical, environmental, and materials chemistry, tBMA-d9 functions as a robust tracer for monitoring degradation, release kinetics, or polymer breakdown pathways.
6. Internal Standard for Analytical Chemistry
The isotopic separation ensures accurate calibration for LC-MS, GC-MS, and quantitative NMR assays, supporting method validation and quality control testing.
FEATURES & BENEFITS
-
High deuterium enrichment for superior isotopic clarity
-
Excellent compatibility with controlled radical polymerization systems
-
Stable, well-defined monomer suitable for reproducible synthesis
-
Enables precise mechanistic and kinetic analysis in polymer science
-
Clear mass spectral and NMR differentiation from native tBMA
-
Ideal for academic, industrial, and pharmaceutical R&D applications
TECHNICAL INSIGHTS FOR FORMULATION & SYNTHESIS
Deuterated tert-Butyl methacrylate-d9 behaves identically to its non-deuterated counterpart in free-radical polymerization, including peroxide-initiated and photoinitiated systems. In advanced controlled polymerization frameworks (ATRP, RAFT, NMP):
-
It allows researchers to quantify isotopic incorporation,
-
Distinguish end-group fidelity,
-
And analyze molecular weight growth with unparalleled accuracy.
The tert-butyl protecting group also offers synthetic versatility. After polymerization, the t-butyl ester can be selectively cleaved to generate methacrylic acid units—retaining deuterium labeling on the polymer backbone and enabling secondary functionalization or crosslinking.
Learn more through,
Deuterated Polymers: A Cornerstone Guide to Synthesis, Applications, and Future Trends
Availability of All the Deuterated Chemicals at ResolveMass Laboratories Inc.
ResolveMass Laboratories: Leading Deuterated Chemical Synthesis Company in the United States.
Deuterated Internal Standards for LC-MS: Selection & Custom Synthesis
How to Choose the Right Deuterated Labelled Chemical Synthesis Company in Canada
How to Choose the Right Deuterium Labelled Compounds Supplier for Your Lab
Deuterium-Labelled Compounds — Synthesis, Applications & Ordering
Custom Synthesis of Deuterated Chemicals
Reviews
There are no reviews yet.