OVERVIEW
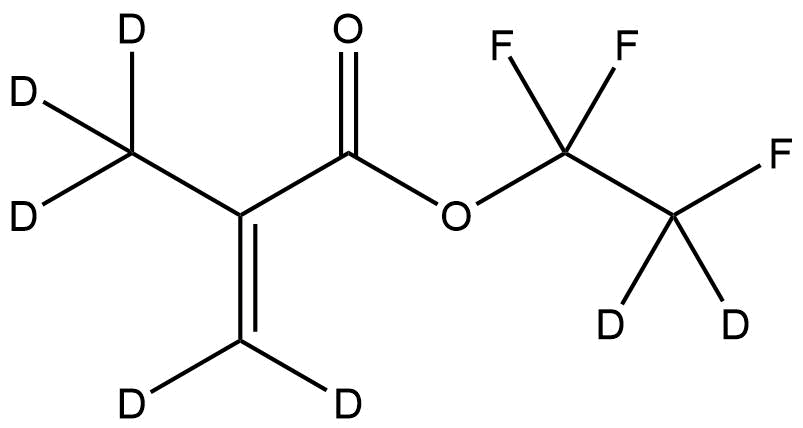
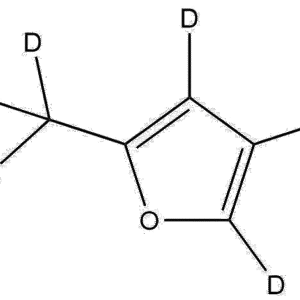
Deuterated Trifluoroethyl methacrylate-d7 is a highly specialized, isotopically labeled methacrylate monomer designed for use in analytical chemistry, polymer synthesis, and mechanistic studies. By replacing exchangeable hydrogen atoms with deuterium (²H), this material provides improved spectroscopic resolution, enhanced NMR tracking capabilities, and reduced background interference in complex analytical workflows. TFEMA-d7 is widely used in laboratories focused on polymer research, controlled radical polymerization (CRP), kinetic isotope effect (KIE) studies, and traceable labeling in advanced materials science.
Its unique combination of a fluorinated side chain and fully deuterated methylene/methyl groups offers a dual advantage: strong electron-withdrawing properties and a spectroscopically distinguishable isotopic signature. These features make this monomer ideal for research environments requiring precision, reproducibility, and molecular-level tracking.
CHEMICAL INFORMATION
-
Chemical Name: Trifluoroethyl Methacrylate-d7
-
Isotopic Labeling: 7 deuterium atoms (²H)
-
Typical Purity: ≥98% (chemical), ≥99% D-isotopic enrichment
-
Functional Group: Methacrylate ester
-
Molecular Features:
-
Strong –CF₃ electron-withdrawing group
-
Deuterated methacrylate backbone
-
High NMR visibility and minimal proton signal interference
-
Deuterated TFEMA retains the reactive vinyl functionality of methacrylates while providing isotopic differentiation, making it suitable for incorporation into copolymers, block architectures, and surface-modified materials.
KEY PROPERTIES
-
Enhanced NMR Clarity:
The deuterium-enriched structure reduces proton-based overlapping signals, enabling precise quantification and structural monitoring in ¹H, ²H, and ¹⁹F NMR studies. -
Excellent Chemical Stability:
The trifluoroethyl group imparts superior resistance against hydrolysis, oxidation, and thermal degradation, essential for demanding polymerization conditions. -
High Reactivity:
Maintains strong compatibility with radical polymerization mechanisms including ATRP, RAFT, and free-radical processes, enabling advanced material design. -
Ideal for Labeling Studies:
Isotopic enrichment enables tracking of monomer distribution in copolymers, monitoring degradation pathways, and performing kinetic isotope effect studies.
APPLICATIONS of Deuterated Trifluoroethyl methacrylate-d7
1. Polymer Synthesis and Advanced Materials
TFEMA-d7 is frequently used in the synthesis of fluorinated or isotopically labeled polymers for:
-
Drug delivery systems
-
Surface coatings
-
Adhesives and sealants
-
Microfluidic and biomedical device components
The presence of fluorine improves hydrophobicity, chemical resistance, and dielectric properties, while deuteration allows analytical monitoring of polymer formation and performance.
2. NMR and Spectroscopic Analysis
The monomer is ideal for:
-
Calibration standards
-
Diffusion studies
-
Polymer chain-growth tracking
-
Determining microstructure and conversion in real time
Deuterium’s minimal interference in proton NMR provides clean spectra, while ¹⁹F NMR benefits from the CF₃ group’s strong signal, offering a dual-mode analytical advantage.
3. Mechanistic and Kinetic Investigations
Scientists use TFEMA-d7 to examine:
-
Radical propagation rates
-
Chain transfer mechanisms
-
Crosslinking kinetics
-
Kinetic isotope effects
This is critical for optimizing polymerization protocols and developing predictive models for material behavior.
4. Degradation and Environmental Pathway Studies
Tracing polymer degradation pathways is simplified using deuterated monomers. Researchers analyze:
-
Environmental breakdown
-
Hydrolysis mechanisms
-
Oxidative stability
-
Microstructural changes after long-term use
The isotopic label provides an unambiguous signature during LC–MS, GC–MS, and NMR analysis of degradation fragments.
BENEFITS FOR RESEARCHERS AND FORMULATORS
-
Precise Analytical Tracking: Perfect for multinuclear NMR and MS workflows.
-
Consistent Polymerization Behavior: Maintains the reactivity profile of non-deuterated analogs while adding isotopic advantages.
-
Enhanced Insight into Polymer Architecture: Enables evaluation of monomer sequence distribution, copolymer ratios, and conversion kinetics.
-
Compatible with Modern Polymerization Techniques: Works smoothly with ATRP, RAFT, and other controlled radical polymerization methods, aiding in the development of well-defined materials.
-
Valuable for Regulatory and Traceability Studies: Isotopic labeling aids in verifying polymer origins, tracking impurities, and enhancing traceability in high-value applications.
Learn more through,
Deuterated Polymers: A Cornerstone Guide to Synthesis, Applications, and Future Trends
Availability of All the Deuterated Chemicals at ResolveMass Laboratories Inc.
ResolveMass Laboratories: Leading Deuterated Chemical Synthesis Company in the United States.
Deuterated Internal Standards for LC-MS: Selection & Custom Synthesis
How to Choose the Right Deuterated Labelled Chemical Synthesis Company in Canada
How to Choose the Right Deuterium Labelled Compounds Supplier for Your Lab
Deuterium-Labelled Compounds — Synthesis, Applications & Ordering
Custom Synthesis of Deuterated Chemicals
Reviews
There are no reviews yet.