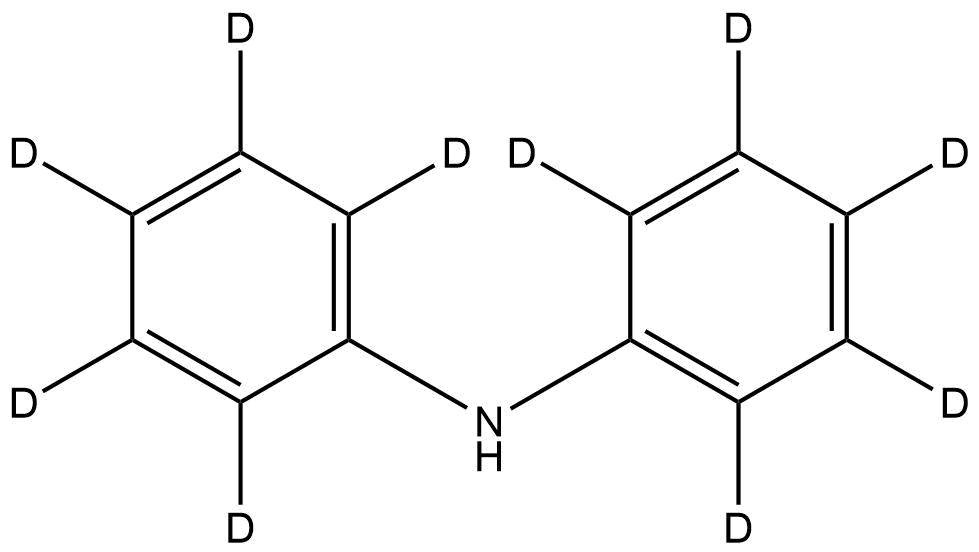
OVERVIEW of Diphenyl-d10-amine | CAS 37055-51-9
Diphenyl-d10-amine is an aromatic secondary amine that serves as a fully deuterated form of diphenylamine, a compound commonly employed in organic synthesis, analytical calibration, and polymer stabilization studies. The replacement of hydrogen with deuterium atoms provides a +10 mass unit shift, making it ideal for isotope-dilution analysis, tracer experiments, and structural elucidation through NMR and MS techniques.
At ResolveMass Laboratories Inc., Diphenyl-d10-amine is synthesized under stringent isotopic labeling conditions, ensuring isotopic enrichment of ≥98 atom % D. The compound’s exceptional stability and purity make it a benchmark reference material for laboratories conducting high-precision chemical, environmental, and pharmaceutical analyses.
CHEMICAL INFORMATION
-
Chemical Name: Diphenyl-d10-amine
-
Synonyms: N-Phenyl-d5-aniline-d5; Deuterated diphenylamine; DPA-d10
-
CAS Number: 37055-51-9
-
Molecular Formula: C₁₂D₁₀NH
-
Molecular Weight: 179.29 g/mol
-
Isotopic Enrichment: ≥ 98 atom % D
-
Chemical Structure: Consists of a nitrogen atom bonded to two phenyl-d5 rings; all aromatic hydrogens replaced by deuterium.
DESCRIPTION AND PROPERTIES
Diphenyl-d10-amine is a stable, non-volatile, and highly pure aromatic amine, uniquely characterized by full deuterium substitution on both phenyl rings. The isotopic substitution increases its molecular mass and alters vibrational spectra slightly, without affecting the chemical or physical behavior compared to its non-deuterated counterpart.
Its strong aromatic stability and non-polar nature make it a valuable internal standard for organic compounds in GC-MS and LC-MS applications. In NMR spectroscopy, deuterium substitution significantly reduces proton signal interference, providing clearer, well-resolved spectra for molecular analysis.
APPLICATIONS of Diphenyl-d10-amine | CAS 37055-51-9
-
Analytical Standard and Internal Calibrant:
Diphenyl-d10-amine is widely used as a stable isotope-labeled internal standard for quantitative mass spectrometry, enabling accurate correction of matrix effects and instrumental fluctuations. Its predictable mass shift (+10 Da) ensures precise identification and quantification in complex analytical matrices. -
Isotope Labeling and Tracer Studies:
The deuterated structure makes it an ideal tracer for studying chemical transformations, degradation pathways, and environmental dispersion of aromatic amines and related compounds. It serves as a non-radioactive, stable alternative to radiolabeled tracers. -
NMR Spectroscopy Applications:
In NMR studies, deuterated aromatic compounds minimize background proton signals, improving spectral resolution. Diphenyl-d10-amine is also used as a reference for chemical shift calibration and solvent suppression experiments. -
Environmental and Forensic Analysis:
Due to its structural similarity to environmental contaminants and antioxidants, it is used as a reference marker for detecting diphenylamine and related compounds in environmental samples and forensic investigations. -
Polymer and Additive Studies:
In polymer chemistry, diphenylamine analogs act as stabilizers against oxidation. The deuterated variant aids in tracing additive migration, degradation behavior, and polymer stabilization mechanisms in analytical research. -
Reaction Mechanism and Kinetic Isotope Effect Studies:
The presence of deuterium provides valuable insight into reaction kinetics, bond cleavage dynamics, and isotope effects, helping chemists understand reaction pathways at the molecular level.
ANALYTICAL ADVANTAGES
-
High isotopic enrichment (≥98 atom % D) ensuring distinct mass spectral separation
-
Ideal as an internal standard for GC-MS, LC-MS, and IR analyses
-
Enhanced accuracy and reproducibility in isotope-dilution quantification
-
Reduced background noise and interference in NMR spectra
-
Chemically stable and compatible with a wide range of analytical solvents
-
Non-radioactive, safe, and environmentally stable labeling compound
QUALITY AND MANUFACTURING
At ResolveMass Laboratories Inc., Diphenyl-d10-amine is synthesized through selective deuterium exchange and advanced organic synthesis routes to ensure complete isotopic substitution and high chemical purity. Each production batch is validated by GC-MS, NMR spectroscopy, and elemental analysis to confirm isotopic labeling, purity, and molecular structure.
We maintain rigorous quality assurance standards that align with international analytical and research-grade requirements. Our deuterated reference compounds provide scientists with consistent, traceable, and reproducible materials for high-accuracy research in analytical chemistry, life sciences, and materials development.
CONCLUSION
Diphenyl-d10-amine is a premium-grade deuterated aromatic amine designed for advanced isotope labeling, mass spectrometry, and spectroscopic applications. Its high isotopic enrichment, superior chemical stability, and purity make it a dependable reference material across analytical, environmental, and industrial research fields. With the precision manufacturing expertise of ResolveMass Laboratories Inc., this compound ensures the consistency and accuracy essential for high-performance analytical and scientific investigations.
Learn more about,
Deuterated Polymers: A Cornerstone Guide to Synthesis, Applications, and Future Trends
Availability of All the Deuterated Chemicals at ResolveMass Laboratories Inc.
ResolveMass Laboratories: Leading Deuterated Chemical Synthesis Company in the United States.
Deuterated Internal Standards for LC-MS: Selection & Custom Synthesis
How to Choose the Right Deuterated Labelled Chemical Synthesis Company in Canada
How to Choose the Right Deuterium Labelled Compounds Supplier for Your Lab
Deuterium-Labelled Compounds — Synthesis, Applications & Ordering
Custom Synthesis of Deuterated Chemicals
Reviews
There are no reviews yet.