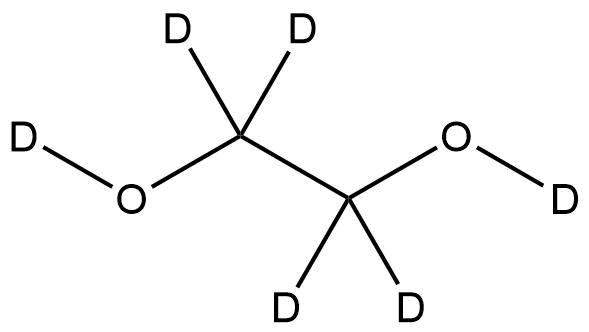
OVERVIEW of Ethylene glycol-d6 | CAS 15054-86-1
Ethylene glycol-d6 (CAS 15054-86-1) is a fully deuterated form of ethylene glycol, in which all six hydrogen atoms are replaced with deuterium. This isotopically labeled compound is primarily utilized as a stable isotope standard in NMR spectroscopy, mass spectrometry, and tracer studies. Owing to its chemical identity with non-deuterated ethylene glycol, it serves as an ideal reference material for metabolic research, hydrogen–deuterium exchange experiments, and isotopic quantification. The high isotopic purity of Ethylene glycol-d6 ensures minimal background interference, providing precise analytical results.
CHEMICAL INFORMATION
-
Name: Ethylene glycol-d6
-
Molecular Formula: C₂D₆O₂
-
Molecular Weight: 68.10 g/mol
-
CAS Number: 15054-86-1
-
Isotopic Labeling: Fully deuterated (six deuterium atoms)
-
Chemical Class: Deuterated diol; stable isotope-labeled compound
-
Stability: Stable under ambient conditions; hygroscopic in nature
APPLICATIONS of Ethylene glycol-d6 | CAS 15054-86-1
-
NMR Spectroscopy:
Used as a deuterated standard or solvent for ¹H and ²H NMR analyses, minimizing proton background and improving signal clarity in complex mixtures.
-
Mass Spectrometry (MS):
Functions as a stable isotope-labeled internal standard for quantitative determination of ethylene glycol and other polyols in biochemical and environmental samples.
-
Tracer and Metabolic Studies:
Employed in metabolic flux analysis and bio-distribution studies to trace the oxidation and degradation pathways of ethylene glycol and its metabolites.
-
Kinetic Isotope Effect (KIE) Studies:
Useful for evaluating reaction mechanisms and hydrogen/deuterium exchange kinetics in chemical and biochemical systems.
-
Calibration & Standardization:
Serves as a reference compound for isotopic calibration and quantitative analytical procedures in research laboratories.
ADVANTAGES of Ethylene glycol-d6 | CAS 15054-86-1
-
Fully deuterated (≥ 98 atom % D) ensuring exceptional isotopic enrichment.
-
Provides minimal proton interference in NMR and MS applications.
-
Chemically equivalent to native ethylene glycol, ensuring consistent reaction behavior.
-
High thermal and chemical stability for long-term storage and usage.
-
Non-radioactive, safe, and compatible with a wide range of analytical instruments.
HANDLING, STORAGE & SAFETY
-
Handling: Handle under dry conditions; avoid contact with strong oxidizing agents.
-
Storage: Keep sealed in a cool (2–8 °C), dry, and dark environment.
-
Safety: May cause mild skin or eye irritation; handle using appropriate protective equipment.
-
Disposal: Dispose of according to institutional chemical waste management protocols.
QUALITY & SPECIFICATION
-
Chemical Purity: ≥ 98 %
-
Isotopic Enrichment: ≥ 98 atom % D
-
Physical State: Viscous liquid
-
Analytical Verification: Confirmed by ¹H NMR, ²H NMR, and mass spectrometry
-
Documentation: Certificate of Analysis (CoA) provided with each batch
SUMMARY
Ethylene glycol-d6 (CAS 15054-86-1) is a fully deuterated isotopologue of ethylene glycol widely used in spectroscopic, analytical, and metabolic research. Its high isotopic purity ensures precise quantitative and qualitative analysis in NMR and MS applications. As a stable, non-radioactive reagent, Ethylene glycol-d6 is an essential material for advanced isotopic studies, metabolic pathway investigations, and reaction mechanism elucidation in both academic and industrial research settings.
Learn more about,
Deuterated Polymers: A Cornerstone Guide to Synthesis, Applications, and Future Trends
Availability of All the Deuterated Chemicals at ResolveMass Laboratories Inc.
ResolveMass Laboratories: Leading Deuterated Chemical Synthesis Company in the United States.
Deuterated Internal Standards for LC-MS: Selection & Custom Synthesis
How to Choose the Right Deuterated Labelled Chemical Synthesis Company in Canada
How to Choose the Right Deuterium Labelled Compounds Supplier for Your Lab
Deuterium-Labelled Compounds — Synthesis, Applications & Ordering
PubChem CID
Reviews
There are no reviews yet.