OVERVIEW of Piperazine-2,2,3,3,5,5,6,6-d8 2 HCl | CAS 848482-21-9
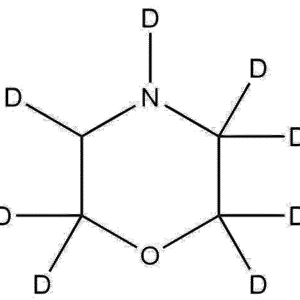
Piperazine-2,2,3,3,5,5,6,6-d₈·2HCl (CAS 848482-21-9) is a fully deuterated dihydrochloride salt of piperazine, containing eight deuterium atoms substituted for all the ring hydrogens. This compound combines the stability and isotopic labeling advantages of deuterium substitution with the chemical reactivity and solubility benefits of the hydrochloride form. It is primarily used in analytical chemistry, pharmaceutical development, and isotopic tracer studies, where high isotopic enrichment and reproducible quantification are essential.
CHEMICAL INFORMATION
-
Name: Piperazine-2,2,3,3,5,5,6,6-d₈ dihydrochloride
-
Molecular Formula: C₄D₈N₂·2HCl
-
Molecular Weight: 167.10 g/mol
-
CAS Number: 848482-21-9
-
Isotopic Enrichment: ≥ 98 atom % D
-
Chemical Class: Deuterated heterocyclic amine (hydrochloride salt)
-
Solubility: Freely soluble in water, methanol, and ethanol; slightly soluble in nonpolar solvents
APPLICATIONS of Piperazine-2,2,3,3,5,5,6,6-d8 2 HCl | CAS 848482-21-9
-
Mass Spectrometry (MS):
Piperazine-2,2,3,3,5,5,6,6-d₈·2HCl serves as an internal standard in quantitative MS analyses. Its deuterium enrichment allows precise isotopic differentiation from native piperazine, making it ideal for calibration and quantification of piperazine-based drug metabolites.
-
NMR Spectroscopy:
The compound is used to reduce proton interference and enhance signal clarity in NMR spectroscopy. The dihydrochloride form ensures good solubility in aqueous solutions, facilitating reliable spectral analysis.
-
Pharmaceutical Research:
Extensively employed in pharmacokinetic and metabolic studies, this compound allows researchers to track the metabolic fate and bioavailability of piperazine derivatives in biological systems.
-
Isotope Tracing and Mechanistic Studies:
Deuterium substitution provides insights into reaction mechanisms, enzyme interactions, and metabolic transformations involving piperazine-based compounds. It is particularly useful in hydrogen–deuterium exchange experiments and stability assessments.
-
Synthesis of Deuterated Intermediates:
Used as a precursor for synthesizing deuterated pharmaceutical intermediates and standards for drug purity testing, metabolic profiling, and reference compound generation.
ADVANTAGES of Piperazine-2,2,3,3,5,5,6,6-d8 2 HCl | CAS 848482-21-9
-
Contains eight deuterium atoms for complete ring labeling.
-
Enhanced solubility and stability in aqueous systems due to dihydrochloride form.
-
High isotopic enrichment ensures superior accuracy in quantitative analysis.
-
Non-radioactive, stable, and safe for laboratory use.
-
Maintains identical chemical reactivity to native piperazine, enabling direct comparison.
HANDLING AND SAFETY
-
Handling: Avoid breathing dust or vapors; use in a well-ventilated area. Prevent contact with skin and eyes.
-
Protective Equipment: Wear chemical-resistant gloves, lab coat, and protective eyewear.
-
Storage: Keep tightly closed in a cool, dry place, away from strong bases and oxidizing agents.
-
Stability: Stable under normal laboratory conditions. Hygroscopic—protect from moisture.
-
Disposal: Dispose of in accordance with local environmental and institutional chemical waste regulations.
SUMMARY
Piperazine-2,2,3,3,5,5,6,6-d₈·2HCl (CAS 848482-21-9) is a high-purity, fully ring-deuterated piperazine dihydrochloride used across analytical, pharmaceutical, and biochemical research. Its isotopic enrichment, stability, and aqueous solubility make it an excellent reference standard for LC-MS/MS, NMR analysis, and metabolic pathway studies. This compound provides an essential tool for accurate quantitation, mechanistic evaluation, and isotopic tracing in modern scientific investigations.

Reviews
There are no reviews yet.