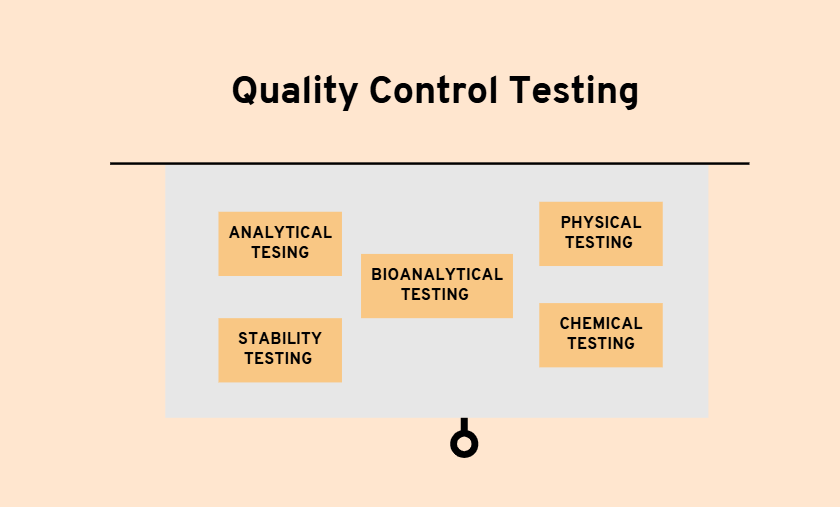
Quality control (QC) testing is the backbone of ensuring the safety, efficacy, and compliance of products across industries such as pharmaceuticals, food, cosmetics, and manufacturing. In the United States, stringent regulatory requirements have made quality control a non-negotiable aspect of product development. Contract testing labs play a pivotal role in ensuring products meet these high standards, offering businesses expertise, efficiency, and regulatory compliance.
This blog explores the role of contract testing labs in QC testing, their benefits, and their growing importance in the U.S. market.
What is Q.C testing in United States?
Quality control testing involves verifying that a product adheres to specified standards of safety, functionality, and compliance. In industries like pharmaceuticals and food manufacturing, QC testing ensures products are free from harmful contaminants and meet regulatory benchmarks set by agencies like the FDA (Food and Drug Administration) and EPA (Environmental Protection Agency).
In the U.S., QC testing is a crucial step in product development and post-production monitoring. It mitigates risks, ensures customer safety, and protects brands from costly recalls and reputational damage.
The Role of Contract Testing Labs in Q.C testing in United States
Contract testing labs have emerged as indispensable partners for companies looking to maintain stringent quality standards without investing heavily in internal testing facilities.
1. Expertise Across Industries
Contract labs bring specialized knowledge in various testing techniques, instruments, and regulatory standards. Whether it’s chromatographic analysis for pharmaceuticals or microbial testing for food, these labs are equipped to handle industry-specific requirements.
For example, in the pharmaceutical sector, contract testing labs assist with:
- Dissolution testing
- Stability studies
- Microbial limit testing
- Nitrosamine impurity testing
2. Advanced Analytical Technology
The cost of maintaining state-of-the-art testing equipment can be prohibitive. Contract testing labs often invest in advanced technologies such as:
- High-performance liquid chromatography (HPLC)
- Mass spectrometry (MS)
- Gas chromatography (GC)
- Differential scanning calorimetry (DSC)
Scalability and Cost Efficiency
For small and mid-sized businesses, setting up a dedicated QC lab may not be feasible. Contract testing labs offer a scalable solution, allowing businesses to outsource testing as needed. This not only reduces costs but also enables companies to focus on their core operations.
4. Regulatory Compliance
Navigating the maze of U.S. regulatory requirements can be challenging. Contract labs provide the expertise needed to ensure compliance with agencies like the FDA, EPA, and USDA. They also assist with documentation, audits, and reporting.
For instance, in the environmental sector, labs help monitor pollutants and contaminants in water and soil, ensuring compliance with EPA standards.
Key Industries Benefiting from Contract Testing Labs
1. Pharmaceutical and Biotechnology
Pharmaceutical companies in the U.S. rely on contract labs for rigorous testing during drug development and manufacturing. Services include:
- Active pharmaceutical ingredient (API) analysis
- Impurity profiling
- Stability testing
2. Food and Beverage
Food safety is a top priority in the U.S., with the FDA and USDA enforcing strict standards. Contract labs conduct tests for:
- Pesticide residues
- Heavy metals
- Pathogens like Salmonella and E. coli
3. Environmental Testing
Contract labs play a vital role in monitoring air, water, and soil quality. Services include:
- PFAS testing
- Heavy metal contamination analysis
- Wastewater testing
4. Consumer Products
From cosmetics to electronics, contract labs ensure consumer products meet safety and performance standards. Testing services include:
- Toxicology studies
- Packaging compliance
- Material durability testing
How to Choose the Right Contract Testing Lab?
Selecting the right partner is crucial for ensuring effective QC testing. Here are some factors to consider:
1. Accreditation and Certifications
Ensure the lab is accredited by recognized organizations such as ISO (International Organization for Standardization) or certified by the FDA. Accreditation demonstrates the lab’s adherence to global testing standards.
2. Experience and Expertise
Look for a lab with a proven track record in your industry. Specialized expertise ensures accurate and reliable results.
3. Advanced Technology
A lab equipped with state-of-the-art analytical instruments ensures precision and efficiency in testing.
4. Turnaround Time
Timely results are critical, especially for industries like pharmaceuticals where delays can impact product launches.
5. Regulatory Knowledge
A good contract lab should have in-depth knowledge of U.S. regulatory requirements and assist with documentation and audits.
6. Customer Support
Responsive and knowledgeable customer support is essential for addressing queries and ensuring smooth collaboration.
Advantages of Outsourcing Quality Control Testing
1. Cost Savings
Outsourcing eliminates the need for expensive lab setups, equipment, and personnel.
2. Access to Expertise
Contract labs bring specialized knowledge and experience, ensuring high-quality results.
3. Focus on Core Operations
Outsourcing allows businesses to concentrate on their core competencies while leaving QC testing to experts.
4. Risk Mitigation
Contract labs help identify potential issues early, reducing the risk of product recalls and regulatory penalties.
5. Scalability
Testing needs can vary over time. Contract labs offer flexible solutions to accommodate changing requirements.
Future Trends in Q.C testing in United States
1. Automation and AI
Advanced technologies like automation and artificial intelligence are transforming QC testing. These technologies enhance efficiency and reduce human error.
2. Sustainability
With growing environmental concerns, labs are adopting sustainable practices such as green chemistry and energy-efficient processes.
3. Integration of Big Data
Big data analytics is being used to identify trends, optimize testing processes, and predict potential issues.
4. Personalized Medicine
In the pharmaceutical sector, QC testing is evolving to support the development of personalized medicine, which requires more precise and targeted analysis.
REFERENCES
- Takle GB. Development, Verification, Validation and Qualification of QC Assays: A Contract Testing Lab Perspective. BioProcessing Journal. 2010 Dec 1;9(2).
- Wedlich RC, Webb KJ. Implementing quality agreements at the contract laboratory. Journal of GXP Compliance. 2012 Oct 1;16(4):54.
- Rule JA. Quality control and the defense contract industry (Doctoral dissertation, Boston University).
- Genzen JR. Regulation of laboratory-developed tests: a clinical laboratory perspective. American Journal of Clinical Pathology. 2019 Jul 5;152(2):122-31.
End-to-End Biomarker Bioanalytical Services Offered by a CRO: Capabilities and Expectations
Introduction Biomarker bioanalytical services CRO partnerships have become indispensable in modern pharmaceutical development, providing the…
Regulatory-Compliant Biomarker Bioanalytical Services for FDA and Health Canada Submissions
Introduction Biomarker bioanalytical services for FDA and Health Canada submissions require rigorous compliance with regulatory…
Outsourced Medicinal Chemistry for Startups: Cost, Speed, and Scientific Advantages
🔍 Summary (Key Takeaways) Outsourced medicinal chemistry for startups offers a strategic advantage by minimizing…
Why Early-Stage Biotech Companies Outsource Chemistry Services to Drug Discovery CROs
Introduction Early-stage biotech companies are increasingly adopting Outsourced Chemistry Services for Biotech to stay competitive…
Why Early-Stage Biotech Companies Outsource Bioanalytical Services to CROs
Introduction Outsourced bioanalytical services play a critical role in helping early-stage biotech companies overcome scientific,…
7 Questions to Ask Before Collaborating with a Full Service Drug Discovery CRO
Summary Before partnering with a Full Service Drug Discovery CRO, the following key questions can…