Introduction:
Quality Bioanalysis is the single most critical factor in reducing scientific and regulatory risk during early drug development. Before a molecule reaches clinical trials, sponsors must generate defensible pharmacokinetic (PK), toxicokinetic (TK), and biomarker data through structured bioanalytical services in drug development.
If that data lacks integrity, validation rigor, or regulatory alignment, the entire development program can stall.
Early drug development is inherently high-risk. Attrition rates are significant, timelines are aggressive, and regulatory scrutiny is intense. In this environment, Quality Bioanalysis acts as a safeguard—ensuring that decisions are based on accurate, reproducible, and regulator-ready data.
At ResolveMass Laboratories Inc., our approach integrates regulated workflows described in our bioanalytical laboratory services and regulated bioanalytical services frameworks to proactively de-risk programs.
Summary:
- Quality Bioanalysis ensures reliable PK, TK, and biomarker data for IND-enabling bioanalytical studies.
- It reduces regulatory risk by aligning with validated frameworks described in bioanalytical method validation guidance.
- Early investment in Quality Bioanalysis prevents costly delays, repeat studies, and issues outlined in common bioanalytical mistakes.
- Robust documentation and compliance systems strengthen data integrity in bioanalytical studies.
- Partnering with an experienced bioanalytical CRO like ResolveMass Laboratories Inc. enhances submission success.
1: What Is Quality Bioanalysis and Why Does It Matter?
Quality Bioanalysis refers to scientifically robust, validated, regulatory-compliant analytical testing of drugs and biomarkers in biological matrices. It ensures accurate bioanalytical quantification, reproducibility, traceability, and regulatory acceptance.
It matters because early development decisions—dose selection, safety margins, go/no-go determinations—depend entirely on validated results generated through disciplined bioanalytical services.
For sponsors asking why bioanalysis is important, the answer is simple: it is the evidence regulators trust.
Core Components of Quality Bioanalysis
| Component | Why It Matters |
|---|---|
| Method Development | Built using structured bioanalytical method development and rapid optimization strategies |
| Method Validation | Conducted under formal bioanalytical method validation protocols |
| Sample Integrity | Controlled via stability and handling procedures |
| Data Integrity | Strengthened through systems discussed in data integrity in bioanalytical studies |
| Regulatory Compliance | Required for bioanalytical services for IND/NDA submissions |
Without Bioanalysis, even promising drug candidates can fail due to unreliable data.
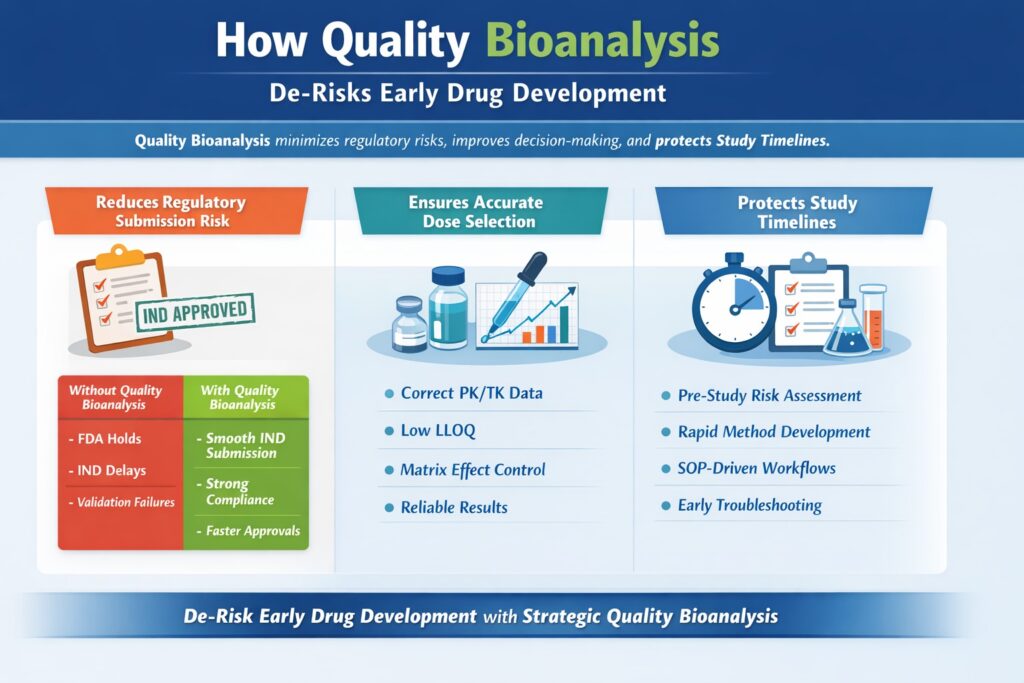
2: How Quality Bioanalysis De-Risks Early Drug Development
Quality Bioanalysis de-risks early drug development by preventing regulatory rejection, minimizing study repetition, ensuring accurate decision-making, and protecting sponsor timelines.
This includes both discovery-phase support and transition to regulated environments, as explained in discovery vs regulated bioanalysis.
Let’s break down how.
1. Quality Bioanalysis Reduces Regulatory Submission Risk
Regulators evaluate bioanalytical data as rigorously as clinical data. Poor validation or weak audit trails often stem from issues described in common bioanalytical mistakes.
Quality Bioanalysis ensures:
- Full compliance with bioanalytical services for IND/NDA submissions
- Accurate reporting for clinical bioanalytical services
- Stability assessments supported by bioanalytical stability testing
- Robust LC-MS/MS workflows such as LC-MS/MS bioanalytical services
Risk Without Quality Bioanalysis:
- FDA clinical hold
- Additional validation studies
- IND delays
- Reputation damage
Risk With Quality Bioanalysis:
- Smooth IND submission
- Reduced agency queries
- Strong reviewer confidence
- Faster clinical progression
2. Quality Bioanalysis Ensures Accurate Dose Selection
Incorrect bioanalytical results can lead to wrong dose selection, increasing safety risks or reducing efficacy.
In early drug development:
- PK profiles guide first-in-human (FIH) dosing
- TK data supports safety margins
- Exposure-response relationships determine therapeutic windows
If analytical sensitivity is insufficient or matrix effects are not controlled, exposure data becomes unreliable.
Quality Bioanalysis ensures:
- Low limits of quantification (LLOQ)
- Proper matrix effect evaluation
- Incurred sample reanalysis (ISR)
- Cross-validation where required
High-quality exposure data is generated through:
- Structured PK/TK bioanalysis
- Dedicated toxicokinetic bioanalysis
- Advanced PK/PD bioanalysis
- Sensitive detection using high-sensitivity bioanalysis
Matrix complexity must be controlled, as discussed in bioanalytical matrix effects.
Accurate data = confident dosing decisions.
3. Quality Bioanalysis Protects Study Timelines
Re-running studies due to analytical failures leads to costly delays. Sponsors increasingly rely on:
- Rapid bioanalytical method development
- Structured bioanalytical method transfer
- Strategic oversight via managing bioanalytical CRO projects
- High-efficiency workflows in high-throughput bioanalysis
Outsourcing strategically to a proven bioanalytical CRO for drug discovery minimizes execution risk.
Common preventable failures include:
- Calibration curve rejection
- Stability failures
- Method reproducibility issues
- Poor sample handling procedures
A robust Quality Bioanalysis framework includes:
- Pre-study risk assessment
- Fit-for-purpose method validation
- Clear SOP-driven workflows
- Early troubleshooting strategies
This proactive approach reduces downstream delays and protects sponsor investments.
3: Quality Bioanalysis and Complex Drug Modalities
Modern therapeutics require advanced analytical strategies.
ResolveMass supports:
- LC-MS bioanalysis for oligonucleotides
- Cell and gene therapy bioanalysis
- Antibody-drug conjugate bioanalytical services
- Proteomics bioanalytical services
- Biosimilar bioanalysis
- Advanced strategies for novel modalities via advanced bioanalytical strategies for complex drug modalities
Each modality demands tailored Quality Bioanalysis workflows.
4: Quality Bioanalysis and Outsourcing Strategy
Strategic outsourcing reduces cost while maintaining compliance when managed properly.
Sponsors explore:
- Bioanalytical outsourcing
- Outsource bioanalysis for biotech startups
- Outsourced bioanalysis for drug development
- Budget planning using bioanalytical testing services cost
- Solutions for emerging companies through affordable bioanalytical services for biotech startups and cost-effective bioanalytical services
A structured virtual bioanalytical strategy enhances oversight without increasing infrastructure costs.
5: Quality Bioanalysis and Data Integrity: Why It’s Non-Negotiable
Data integrity is inseparable from Quality Bioanalysis. Regulators expect ALCOA+ compliance (Attributable, Legible, Contemporaneous, Original, Accurate).
Key safeguards include:
- Secure LIMS systems
- Audit trail reviews
- Controlled access systems
- Independent quality assurance oversight
- Thorough deviation investigations
Incomplete documentation can invalidate otherwise scientifically sound data. That’s why Quality Bioanalysis must integrate scientific rigor with compliance discipline.
6: Quality Bioanalysis in IND-Enabling Studies
IND-enabling studies rely heavily on validated bioanalytical methods to support safety and exposure data.
These studies typically include:
- GLP toxicology studies
- Safety pharmacology studies
- PK/PD modeling
- Biomarker assessments
Bioanalysis supports these programs by:
- Validating methods before GLP study initiation
- Performing cross-validation between species matrices
- Conducting stability studies under study conditions
- Generating regulatory-ready reports
A weak bioanalytical strategy at this stage can derail IND timelines entirely.
7: Key Risk Areas That Quality Bioanalysis Addresses
Quality Bioanalysis proactively identifies and mitigates analytical, regulatory, and operational risks before they impact timelines or submissions. Below is a structured breakdown of the most critical risk areas and how they are controlled.
| Risk Area | How Quality Bioanalysis Mitigates It |
|---|---|
| Matrix Effects | Comprehensive evaluation during method validation, including selectivity testing and matrix factor assessment |
| Sensitivity Gaps | Optimized LC-MS/MS method development to achieve appropriate LLOQ and dynamic range |
| Stability Issues | Freeze-thaw, bench-top, autosampler, and long-term stability testing under real study conditions |
| Documentation Errors | QA review processes, SOP-driven workflows, audit trail verification, and deviation management |
| Regulatory Misalignment | Strict adherence to global bioanalytical validation guidance and IND/NDA submission expectations |
Why Early Risk Identification Matters
By addressing these risks early through structured Quality Bioanalysis, sponsors:
- Prevent regulatory queries and clinical holds
- Avoid repeat validation or reanalysis
- Protect IND timelines
- Improve reviewer confidence
- Reduce overall development cost
Early-stage analytical rigor is far less expensive than late-stage correction. Proactive Quality Bioanalysis transforms potential liabilities into controlled, documented, and regulator-ready outcomes.
8: The Role of Experienced CROs in Delivering Quality Bioanalysis
Experience directly impacts bioanalytical reliability.
An experienced laboratory:
- Anticipates regulatory expectations
- Designs fit-for-purpose validation strategies
- Understands matrix complexity
- Implements advanced LC-MS/MS platforms
- Maintains strong QA oversight
At ResolveMass Laboratories Inc., our scientific team combines regulatory understanding with deep analytical expertise. Our approach to Quality Bioanalysis is built on:
- Advanced LC-MS/MS capabilities
- Proven method transfer workflows
- Cross-validation expertise
- Transparent communication
- Robust quality systems
This combination significantly reduces scientific and regulatory risk.
9: Why Early Investment in Quality Bioanalysis Saves Money
Quality Bioanalysis reduces long-term costs by preventing rework and regulatory setbacks.
While high-quality bioanalysis may seem more resource-intensive initially, it prevents:
- Repeat toxicology studies
- Additional method validation
- Regulatory delays
- Clinical holds
Cost comparison:
| Scenario | Outcome |
|---|---|
| Poor Quality Bioanalysis | Revalidation + delayed IND |
| Strong Quality Bioanalysis | First-pass regulatory success |
The ROI of Bioanalysis becomes clear when timelines and investor confidence are considered.
10: Quality Bioanalysis and AI-Driven Drug Development
As AI-driven discovery expands, validated data becomes even more important. Learn more about AI in bioanalysis.
Clean datasets generated through regulated workflows improve predictive modeling reliability and translational accuracy.
AI models rely on:
- Clean PK datasets
- Accurate biomarker measurements
- Reproducible exposure data
Poor-quality data compromises machine learning outputs. High-quality datasets enhance predictive modeling accuracy and translational reliability.
11: How ResolveMass Laboratories Inc. Embodies Quality Bioanalysis
Quality is not just analytical performance—it is a culture.
At ResolveMass Laboratories Inc., Quality Bioanalysis is reflected in:
- Experienced scientific leadership
- Strong regulatory familiarity
- Rigorous documentation standards
- Transparent sponsor communication
- Commitment to data integrity
Our laboratory environment is designed to support:
- IND-enabling studies
- PK/TK bioanalysis
- Biomarker bioanalysis
- Method transfer and cross-validation
ResolveMass Laboratories Inc. integrates:
- Comprehensive bioanalytical services overview
- Specialized bioanalytical CRO services for PK and TK
- Xenobiotic analysis via LC-MS/MS bioanalysis of xenobiotics
- Strategic problem-solving around challenges in bioanalytical method development
Bioanalysis is embedded into every stage—from discovery to regulated clinical programs.
We partner with biotech innovators to ensure that early development decisions are backed by defensible, regulator-ready data.
Conclusion:
Quality Bioanalysis is not just a laboratory function—it is a strategic safeguard that de-risks early drug development. From accurate dose selection to regulatory submission readiness, it protects sponsor timelines, investor confidence, and patient safety.
Organizations that invest early in Quality Bioanalysis significantly reduce regulatory setbacks, prevent costly rework, and accelerate clinical progression.
At ResolveMass Laboratories Inc., we help biotechs transform analytical data into regulatory confidence.
Frequently Asked Questions:
Bioanalysis in drug development refers to the quantitative measurement of drugs, metabolites, and biomarkers in biological matrices such as plasma, serum, urine, or tissues. It supports pharmacokinetic (PK), toxicokinetic (TK), and exposure-response studies. Bioanalysis ensures accurate dose selection, safety evaluation, and regulatory submission readiness. High-quality bioanalysis is essential for IND-enabling and clinical studies.
Bioinformatics plays a critical role in analyzing large biological datasets to identify drug targets, predict molecular interactions, and optimize lead compounds. It supports genomics, proteomics, and biomarker discovery efforts. In development phases, bioinformatics helps interpret PK/PD relationships and exposure-response modeling. It also enhances AI-driven drug discovery by improving predictive accuracy and translational insights.
A bioassay measures the biological activity or potency of a drug candidate. It evaluates how a drug interacts with its target, such as receptor binding, enzyme inhibition, or cell response. Bioassays are used in early screening, potency determination, stability studies, and biosimilar comparisons. They are especially important for biologics, cell therapies, and antibody-based drugs.
Biomarkers are measurable biological indicators used to assess disease progression, drug response, or safety. They help in patient stratification, dose optimization, and monitoring therapeutic efficacy. Predictive biomarkers guide personalized medicine strategies, while safety biomarkers detect potential toxicity early. Validated biomarkers strengthen regulatory submissions and clinical trial outcomes.
The five pillars of drug discovery are:
1. Target Identification – Identifying a biological pathway or protein linked to disease.
2. Target Validation – Confirming that modulating the target produces therapeutic benefit.
3. Lead Identification – Discovering compounds that interact with the target.
4. Lead Optimization – Improving potency, selectivity, and pharmacokinetic properties.
5. Preclinical Development – Conducting safety, PK, and toxicology studies before clinical trials.
Together, these pillars form the foundation for successful drug development programs.
Reference
- Amit Etkin. Opportunities for use of neuroimaging in de-risking drug development and improving clinical outcomes in psychiatry: an industry perspective.https://www.nature.com/articles/s41386-024-01970-8
- ICH Q12 AT PROGRAM SCALE: EMBEDDING POST-APPROVAL CHANGE
MANAGEMENT INTO PORTFOLIO GOVERNANCE.https://ijetrm.com/issues/files/Sep-2020-19-1758286882-NOV202012.pdf - John S. Kendrick & Colin Webber. One small step in time, one giant leap for DMPK kind – a CRO perspective of the evolving core discipline of drug development.https://www.tandfonline.com/doi/abs/10.1080/00498254.2022.2124389
- Cromwel Tepap Zemnou.Integrating Computational Biology in Modern Drug Discovery: A Synergistic Approach of Structure-Based, Ligand-Based, and Network Pharmacology Strategies.https://www.preprints.org/manuscript/202601.2300

