.
.
.
Stability Studies for Pharma

Stability Studies for Pharma are a critical regulatory and scientific requirement to ensure the safety, quality, and efficacy of pharmaceutical products throughout their shelf life. At ResolveMass Laboratories Inc., we provide scientifically robust, regulatory-compliant stability testing services that support drug development, regulatory submissions, and lifecycle management for pharmaceutical companies worldwide. When degradation pathways or product vulnerabilities need deeper investigation, our teams often integrate advanced analytical support such as forced degradation studies to strengthen stability strategies.
With deep expertise in analytical science and a strong understanding of global regulatory expectations, our stability programs are designed to deliver accurate, reproducible, and audit-ready data you can trust. These programs are further supported by sophisticated techniques including liquid chromatography–mass spectrometry (LC-MS) analysis for precise impurity profiling over time.
What Are Stability Studies in the Pharmaceutical Industry?
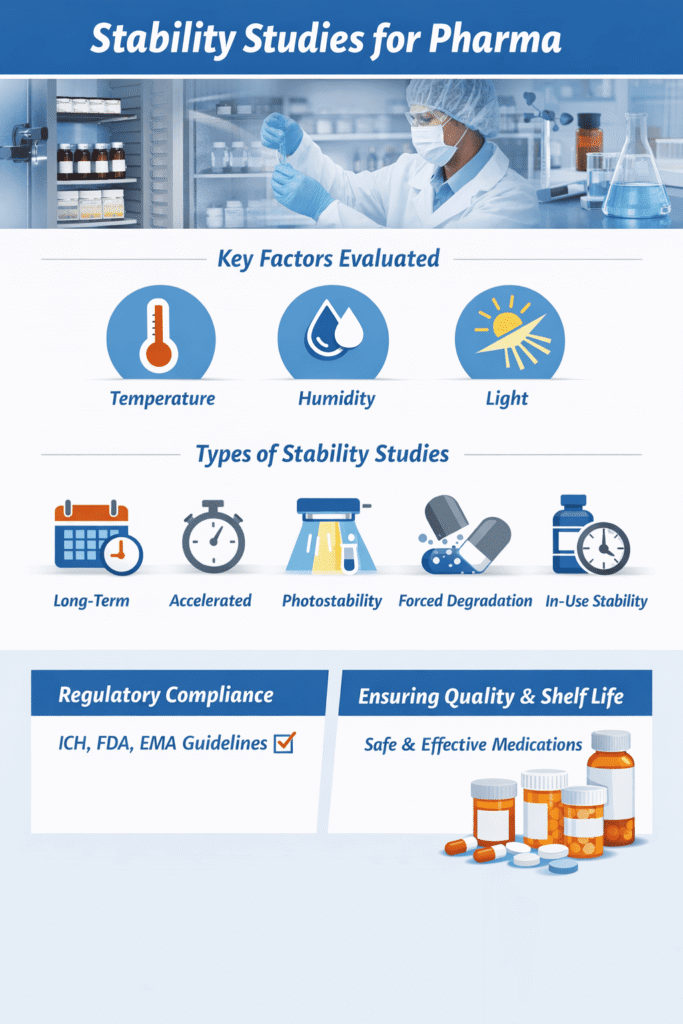
Stability studies evaluate how the quality of a pharmaceutical product varies over time under the influence of environmental factors such as temperature, humidity, and light. These studies establish shelf life, retest periods, storage conditions, and packaging suitability for drug substances and drug products. In cases where trace-level impurities or emerging risks are detected, targeted assessments like nitrosamine analysis can play a crucial role in ensuring patient safety.
Regulatory authorities including the FDA, EMA, and ICH require stability data to demonstrate that a product remains within approved specifications from manufacture through expiration. High-confidence identification of degradation products is often achieved using high-resolution mass spectrometry (HRMS) analysis as part of a comprehensive stability program.
Why Stability Studies for Pharma Are Critical
- Demonstrate product safety and efficacy over time
- Support IND, NDA, ANDA, and MAA submissions
- Define shelf life and storage conditions
- Ensure compliance with ICH Q1A(R2), Q1B, Q1C, Q1D, and Q1E
- Reduce regulatory risk and costly product recalls
- Support post-approval changes and lifecycle management
Stability Studies Services at ResolveMass Laboratories Inc.
ResolveMass Laboratories Inc. offers end-to-end stability studies for pharma, backed by advanced analytical capabilities, validated methods, and experienced scientific oversight. Our studies are executed in compliance with global regulatory guidelines and designed to meet both early-phase development and commercial requirements. Depending on product characteristics, complementary techniques such as GC-FID and GC-MS–based analytical services may be incorporated to monitor volatile or residual components throughout stability testing.
Types of Stability Studies We Offer
- Long-Term Stability Studies
- Accelerated Stability Studies
- Intermediate Stability Studies
- Photostability Studies (ICH Q1B)
- Forced Degradation Studies
- In-Use Stability Studies
- Ongoing / Commitment Stability Programs
Pharmaceutical Products We Support
Our stability studies are designed for a wide range of pharmaceutical dosage forms and drug substances, including:
- Small-molecule drug substances (API)
- Solid oral dosage forms (tablets, capsules)
- Parenterals and injectables
- Topical and transdermal formulations
- Liquid oral formulations
- Modified-release products
- Combination products
Regulatory-Compliant Stability Chambers & Infrastructure
ResolveMass Laboratories Inc. operates qualified and continuously monitored stability chambers capable of supporting ICH, FDA, EMA, and WHO climatic zones. Our infrastructure ensures precise environmental control, real-time monitoring, alarm systems, and documented traceability for every study.
All stability chambers are:
- IQ/OQ/PQ qualified
- Continuously monitored with backup systems
- Maintained under GMP conditions
- Fully audit-ready for regulatory inspections
Expertise You Can Rely On
Our stability studies are overseen by experienced pharmaceutical scientists with extensive backgrounds in analytical chemistry, formulation development, and regulatory compliance. Each study is scientifically justified, risk-based, and aligned with the specific regulatory pathway of your product.
We work as a true scientific partner, providing data interpretation, trend analysis, and technical support—not just raw results.
Data Integrity, Accuracy & Trust
At ResolveMass Laboratories Inc., data integrity is non-negotiable. Our stability studies follow strict quality systems to ensure:
- ALCOA+ compliant data practices
- Validated analytical methods
- Robust change control and deviation management
- Secure data storage and traceability
- Transparent reporting and documentation
This commitment ensures your stability data stands up to regulatory scrutiny with confidence.
Why Choose ResolveMass Laboratories Inc. for Stability Studies for Pharma?
- Proven expertise in pharmaceutical stability testing
- Regulatory-driven study design
- Advanced analytical capabilities
- Flexible programs for early-phase to commercial products
- Strong quality culture and audit readiness
- Clear communication and scientific accountability
Frequently Asked Questions
Stability studies in pharma are scientific tests performed to determine how a drug substance or drug product maintains its quality over time. These studies assess the impact of environmental factors such as temperature, humidity, and light. The goal is to establish shelf life, storage conditions, and expiration dating to ensure patient safety.
The primary ICH guideline for stability studies is ICH Q1A(R2), which outlines requirements for stability testing of new drug substances and products. Additional guidelines like Q1B, Q1C, Q1D, and Q1E address photostability, new dosage forms, bracketing/matrixing, and data evaluation. Together, they provide a global regulatory framework.
There are several types of stability studies, with the most common being long-term, accelerated, and intermediate studies. Other important types include photostability, forced degradation, in-use stability, and ongoing stability studies. Each type serves a specific purpose depending on the product’s development stage and regulatory needs.
The ICH stability zones classify global climatic conditions into five zones: Zone I (temperate), Zone II (subtropical), Zone III (hot and dry), Zone IVa (hot and humid), and Zone IVb (hot and very humid). These zones define the storage conditions required for stability testing based on geographic regions.
An ICH stability study is a structured testing program conducted according to International Council for Harmonisation guidelines. It evaluates how pharmaceutical products behave under defined environmental conditions over time. The data generated supports regulatory submissions and helps establish shelf life and labeling claims.
Stability study parameters typically include appearance, assay, degradation products, dissolution, moisture content, and microbial limits where applicable. Physical, chemical, and sometimes microbiological attributes are monitored at predefined intervals. These parameters ensure the product remains within approved specifications throughout its shelf life.
Conclusion
When it comes to Stability Studies for Pharma, choosing the right laboratory directly impacts regulatory success, product quality, and patient safety. ResolveMass Laboratories Inc. delivers scientifically rigorous, compliant, and reliable stability studies that support your product from development through commercialization.
Contact our team today to discuss a customized stability strategy built on expertise, integrity, and trust.
Reference
- Sultana, S., & Mohammed, S. (2018). A review on stability studies of pharmaceutical products. International Journal for Pharmaceutical Research Scholars (IJPRS), 7(1). Retrieved from https://www.ijprs.com/wp-content/uploads/2018/09/IJPRS-V7-I1-00003.pdf
- Zothanpuii, F., Rajesh, R., & Selvakumar, K. (2020). A review on stability testing guidelines of pharmaceutical products. Asian Journal of Pharmaceutical and Clinical Research, 13(10), 3–9. https://journals.innovareacademics.in/index.php/ajpcr/article/download/38848/23690
- Shelar, S. S., Pagar, P. S., & Wagh, M. K. (2025). Stability studies in pharmaceuticals: Guidelines and recent advances. International Journal of Scientific Development and Research (IJSDR), 10(4). Retrieved from https://ijsdr.org/papers/IJSDR2504037.pdf
- Mani, S., Arunachalam, A., & Shankar, M. (2013). Stability studies: A review. Retrieved from https://www.researchgate.net/publication/318877092_STABILITY_STUDIES_A_REVIEW