
Introduction:
Toxicokinetic bioanalysis is the cornerstone of preclinical safety assessment, providing essential data on how test substances behave in living organisms during toxicity studies. This specialized field combines toxicology principles with pharmacokinetic analysis to ensure that new pharmaceutical compounds, biologics, and chemicals undergo rigorous safety evaluation before human exposure.
In modern drug development, understanding the relationship between administered dose and systemic exposure is not optional—it is a regulatory expectation. Toxicokinetic (TK) testing bridges the gap between toxicology findings and exposure levels, enabling informed safety decisions prior to clinical trials. These studies rely heavily on robust bioanalytical laboratory services supported by validated LC-MS/MS workflows.
At ResolveMass Laboratories Inc., toxicokinetic bioanalysis is supported by deep expertise across bioanalytical services in drug development, from early discovery to IND-enabling studies.
Summary:
Key Takeaways:
- Toxicokinetic bioanalysis measures drug absorption, distribution, metabolism, and elimination in preclinical safety studies
- TK testing is mandatory for regulatory submissions to FDA, EMA, and other global health authorities
- Advanced LC-MS/MS bioanalysis of xenobiotics enables high-sensitivity exposure measurement
- Proper TK study design integrates seamlessly with toxicology protocols to optimize safety assessments
- Comprehensive TK data helps predict human pharmacokinetics and identify potential safety concerns early
- Validated bioanalytical method development and validation ensures regulatory acceptance
- Expert laboratories provide validated bioanalytical methods that meet GLP compliance standards
- TK testing reduces late-stage drug development failures by identifying safety issues during preclinical phases
Do you know how the right bioanalytical CRO can significantly reduce development cost and regulatory risk?
👉 https://resolvemass.ca/bioanalytical-cro/
1: What is Toxicokinetic (TK) Testing?
Toxicokinetic testing is the measurement and characterization of systemic exposure to a test substance during toxicity studies in animals. It answers the fundamental question: what concentration of the drug reaches the bloodstream and target tissues when administered at specific doses?
Unlike standard PK studies, toxicokinetic bioanalysis evaluates exposure at supra-therapeutic levels, often requiring specialized small- and large-molecule bioanalytical services.
Unlike traditional pharmacokinetic studies that focus on therapeutic dosing in healthy subjects, toxicokinetic bioanalysis examines drug behavior at much higher doses used in safety assessment studies. This distinction is crucial because:
- Dose-dependent changes in absorption, metabolism, and elimination may occur at toxicological doses
- Saturation of metabolic pathways can lead to non-linear kinetics
- Accumulation potential becomes apparent with repeated dosing
- Active metabolite formation may contribute to observed toxicity
Key TK Parameters Measured
| Parameter | Definition | Clinical Significance |
|---|---|---|
| Cmax | Maximum plasma concentration | Peak exposure level achieved |
| Tmax | Time to reach maximum concentration | Absorption rate indicator |
| AUC | Area under the concentration-time curve | Total systemic exposure |
| T½ | Elimination half-life | Duration of exposure |
| CL | Clearance | Rate of drug removal |
| Vd | Volume of distribution | Tissue distribution extent |
These complexities are especially relevant when comparing small-molecule vs large-molecule bioanalysis.
2: Why is Toxicokinetic Bioanalysis Essential for Preclinical Studies?
TK testing is mandated by regulatory authorities worldwide because it provides the exposure data necessary to interpret toxicity findings and establish safe human dose ranges. Without comprehensive toxicokinetic data, toxicity studies provide incomplete safety information.
Regulatory Requirements
Global regulatory agencies require TK assessment as part of preclinical safety packages:
- FDA (United States): ICH S3A guidance mandates TK evaluation in repeated-dose toxicity studies
- EMA (European Union): Requires systemic exposure data to support first-in-human dose selection
- PMDA (Japan): Expects thorough TK characterization in all toxicology species
- Health Canada: Requires exposure-response relationships for safety assessment
These expectations align closely with bioanalytical services for IND and NDA submissions
Critical Applications of TK Data
- Dose Selection for Toxicity Studies: TK bioanalysis helps identify doses that achieve target exposure multiples over anticipated human exposure
- Exposure-Response Relationships: Correlating systemic exposure with observed toxicological effects provides more meaningful safety margins than dose-based comparisons alone
- Species Comparison: Understanding exposure differences between toxicology species and humans improves translation of preclinical findings
- Accumulation Assessment: Multiple-dose TK studies reveal whether drugs accumulate with repeated administration, indicating potential long-term safety concerns
- Gender Differences: TK testing identifies sex-based differences in exposure that may necessitate gender-specific dosing strategies
3: Toxicokinetic Study Design and Integration with Toxicology Protocols
Effective TK studies are carefully integrated into toxicology protocols, with sample collection timepoints strategically selected to capture complete exposure profiles without compromising the toxicity assessment. This integration requires careful planning and coordination between toxicologists and bioanalytical scientists.
Typical TK Sampling Strategies
Single-Dose TK Studies:
- Conducted on Day 1 of toxicity studies
- Multiple timepoints post-dose (typically 6-10 samples per animal)
- Captures absorption, distribution, and elimination phases
- Provides baseline exposure characterization
Multiple-Dose TK Studies:
- Conducted at study midpoint and termination
- Assesses steady-state exposure and accumulation
- Typically includes pre-dose (trough) and post-dose samples
- Demonstrates time-dependent changes in pharmacokinetics
This integration often leverages PK/PD bioanalysis and biomarker support to strengthen interpretation.
👉 https://resolvemass.ca/biomarker-bioanalytical-services/
Study Design Considerations
- Species Selection: Rodents (rats, mice) and non-rodents (dogs, non-human primates) based on regulatory requirements
- Sample Size: Typically 3-6 animals per sex per dose level for adequate statistical power
- Sampling Matrix: Plasma is standard, but serum, whole blood, or tissues may be analyzed depending on study objectives
- Analytical Validation: Bioanalytical methods must be fully validated according to regulatory guidelines before sample analysis
4: Advanced Bioanalytical Methods in Toxicokinetic Testing
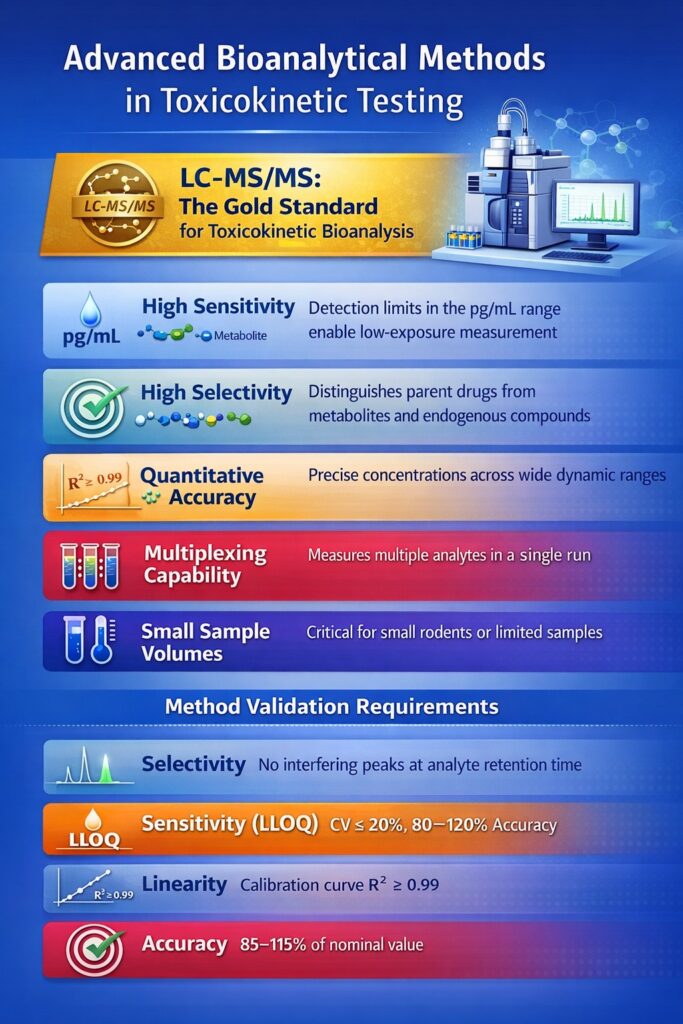
Modern toxicokinetic bioanalysis relies primarily on liquid chromatography-tandem mass spectrometry (LC-MS/MS), which offers unparalleled sensitivity, specificity, and throughput for drug quantification. This technology has revolutionized TK testing by enabling accurate measurement of drugs and metabolites at very low concentrations.
LC-MS/MS: The Gold Standard
LC-MS/MS provides several critical advantages for toxicokinetic bioanalysis:
- High Sensitivity: Detection limits in the pg/mL range enable measurement of low-exposure compounds
- Selectivity: Distinguishes parent drugs from metabolites and endogenous compounds
- Quantitative Accuracy: Provides precise concentration measurements across wide dynamic ranges
- Multiplexing Capability: Simultaneous measurement of multiple analytes in a single run
- Small Sample Volumes: Critical when working with small rodents or limited sample availability
These workflows are supported by:
- Bioanalytical quantification services
- Expertise in bioanalytical matrix effects
- Proven solutions to challenges in bioanalytical method development
Method Validation Requirements
To ensure data quality and regulatory acceptance, bioanalytical methods undergo rigorous validation:
| Validation Parameter | Acceptance Criteria | Purpose |
|---|---|---|
| Selectivity | No interfering peaks at analyte retention time | Ensures measurement specificity |
| Sensitivity (LLOQ) | CV ≤20%, accuracy 80-120% | Defines detection limit |
| Linearity | R² ≥0.99 across calibration range | Ensures accurate quantification |
| Accuracy | 85-115% of nominal concentration | Validates method correctness |
| Precision | CV ≤15% (≤20% at LLOQ) | Demonstrates reproducibility |
| Stability | Established in biological matrix | Confirms sample integrity |
5: Toxicokinetics vs Pharmacokinetics: Key Differences
While PK focuses on therapeutic exposure, toxicokinetic bioanalysis focuses on safety-driven exposure assessment embedded in toxicology studies.
This distinction is particularly important for:
- Gene, cell, and biologic therapies
- High-dose toxicology
- Chronic exposure
| Aspect | Pharmacokinetics (PK) | Toxicokinetics (TK) |
|---|---|---|
| Study purpose | Efficacy and dosing | Safety and risk assessment |
| Study design | Standalone | Embedded in toxicity studies |
| Dose levels | Therapeutic | Supra-therapeutic |
| Regulatory role | IND support | Mandatory for tox interpretation |
6: Interpreting TK Data: From Concentrations to Safety Decisions
Toxicokinetic data interpretation goes beyond simple concentration measurements—it involves comprehensive analysis of exposure-response relationships, interspecies comparisons, and safety margin calculations. Expert scientific interpretation transforms raw analytical data into actionable safety insights.
Calculating Safety Margins
The most common approach compares systemic exposure in toxicology species to anticipated human exposure:
Safety Margin = AUC (NOAEL in animals) / AUC (proposed human dose)
Where NOAEL is the No Observed Adverse Effect Level. Safety margins of 10-fold or greater are typically considered acceptable, though specific requirements vary by drug class and indication.
Key Interpretation Considerations
- Dose Proportionality: Does exposure increase proportionally with dose, or is there evidence of saturation?
- Time-Dependent Changes: Does clearance change with repeated dosing, suggesting enzyme induction or inhibition?
- Metabolite Exposure: Are active or toxic metabolites present at significant levels?
- Exposure Variability: Is there high inter-animal variability that might indicate absorption or metabolic differences?
These analyses often integrate clinical and non-clinical bioanalytical services
7: Quality Standards and GLP Compliance in TK Bioanalysis
Regulatory acceptance of toxicokinetic data requires strict adherence to Good Laboratory Practice (GLP) standards, ensuring data integrity, traceability, and reproducibility. GLP compliance is not optional for studies supporting regulatory submissions.
GLP Requirements for TK Testing
- Study Protocol: Detailed, approved study plan before initiation
- Standard Operating Procedures: Validated procedures for all activities
- Quality Assurance: Independent QA audits of study conduct and data
- Raw Data Retention: Complete records maintained for regulatory inspection
- Study Report: Comprehensive documentation of methods, results, and conclusions
GLP compliance is mandatory for regulatory TK data acceptance. ResolveMass operates within fully regulated environments aligned with:
- Regulated bioanalytical services
- North American regulatory standards
At ResolveMass Laboratories Inc., our GLP-compliant facilities and experienced scientific team ensure that every toxicokinetic study meets the highest quality standards required for regulatory submissions worldwide.
8: Common Challenges in Toxicokinetic Studies and How to Overcome Them
Even with advanced technology, TK testing presents unique challenges that require expert problem-solving and methodological innovation. Understanding these challenges and their solutions is essential for successful study execution.
Challenge 1: Low Exposure Compounds
Solution: Advanced sample preparation techniques, including solid-phase extraction and protein precipitation optimization, combined with highly sensitive MS detection methods, enable quantification of compounds with poor bioavailability.
Challenge 2: Complex Biological Matrices
Solution: Matrix-matched calibration standards and careful method validation address interference from endogenous compounds, lipids, and proteins in biological samples.
Challenge 3: Unstable Analytes
Solution: Rapid sample processing, appropriate stabilizers, and validated stability studies ensure analyte integrity from collection through analysis.
Challenge 4: Active Metabolites
Solution: Comprehensive metabolite identification studies followed by validated bioanalytical methods for major metabolites provide complete exposure characterization.
- These are addressed through bioanalytical outsourcing solutions that combine expertise, scalability, and cost control.
👉 https://resolvemass.ca/bioanalytical-services-outsourcing-for-pharma/ - Cost transparency is supported through clear bioanalytical testing services cost planning.

9: Integration of TK Data with Overall Safety Assessment
Toxicokinetic bioanalysis data doesn’t exist in isolation—it must be integrated with toxicity findings, clinical pathology, and histopathology to provide comprehensive safety assessment. This integration reveals whether observed toxicity correlates with systemic exposure levels.
Building Exposure-Response Relationships
Strong exposure-response relationships provide confidence in:
- Target organ identification: Which organs show toxicity at specific exposure levels?
- Threshold determination: What exposure level produces the first signs of toxicity?
- Reversibility assessment: Do effects resolve when exposure decreases?
- Margin of safety calculation: How much buffer exists between toxic and therapeutic exposures?
High-throughput studies benefit from:
Future Trends in Toxicokinetic Testing
The field of toxicokinetic bioanalysis continues to evolve with technological advances:
- Microsampling Techniques: Reduced blood volumes allow more frequent sampling and improved animal welfare
- PBPK Modeling: Physiologically-based pharmacokinetic models predict human exposure from animal TK data
- Biomarker Integration: Combining TK with pharmacodynamic biomarkers provides deeper mechanistic insights
- Automated Sample Preparation: Robotics and automation increase throughput and reduce variability
Conclusion:
Toxicokinetic bioanalysis is a critical component of preclinical safety assessment, providing the exposure data necessary to interpret toxicity findings and make informed decisions about drug development progression. The complexity of TK testing—from study design through analytical method validation and data interpretation—requires specialized expertise and state-of-the-art capabilities.
At ResolveMass Laboratories Inc., we combine advanced LC-MS/MS technology with deep scientific expertise to deliver high-quality toxicokinetic data that meets regulatory standards. Our GLP-compliant facilities and experienced team ensure that your preclinical studies generate the robust exposure data needed for successful regulatory submissions and safe advancement to clinical trials. We deliver comprehensive bioanalytical services across small molecules, biologics, and advanced therapies
👉 https://resolvemass.ca/resolvemass-bioanalytical-services-overview/
Whether you’re developing small molecules, biologics, or specialty chemicals, comprehensive toxicokinetic testing is essential for understanding safety profiles and protecting future patients. Don’t compromise on the quality of your TK bioanalysis—the success of your development program depends on it.
Frequently Asked Questions:
Toxicokinetic studies in the preclinical stage measure systemic drug exposure during animal toxicity studies to relate dose levels to observed toxic effects.
They help determine how much of a drug reaches the bloodstream and tissues at toxic doses, supporting safety margin assessment before human trials.
A preclinical toxicity test evaluates the potential harmful effects of a drug or chemical in animals before it is tested in humans.
These studies identify target organs of toxicity, safe dose ranges, and potential risks, forming the foundation for regulatory safety assessment.
The four processes of toxicokinetics are Absorption, Distribution, Metabolism, and Excretion (ADME).
Together, they describe how a substance enters the body, spreads to tissues, is chemically transformed, and is eliminated during toxicity studies.
TK (toxicokinetics) in toxicology refers to the study of systemic exposure to a substance during toxicity testing.
It links administered dose to internal exposure, enabling accurate interpretation of toxicological findings based on concentration rather than dose alone.
The four main types of drug toxicity are acute, subacute, chronic, and developmental/reproductive toxicity.
These categories describe toxicity based on exposure duration and the biological systems affected.
A toxicity study in pharmacology investigates the adverse effects of a drug on biological systems to determine its safety profile.
These studies assess dose-related toxic effects, target organs, reversibility, and exposure-response relationships before clinical use.
Reference
- Toxicokinetics in preclinical drug development of small-molecule new chemical entities.https://analyticalsciencejournals.onlinelibrary.wiley.com/doi/abs/10.1002/bmc.5553
- Chapter 16 – Toxicity and toxicokinetic considerations in product development and drug research.https://www.sciencedirect.com/science/chapter/edited-volume/abs/pii/B9780323983679000019
- Chapter 9 – Use of toxicokinetic data in preclinical safety assessment.https://www.sciencedirect.com/science/chapter/edited-volume/abs/pii/B9780443158421000077
- Chapter 8 – Design of toxicokinetic studies.https://www.sciencedirect.com/science/chapter/edited-volume/abs/pii/B9780443158421000065
- Bridging from Preclinical to Clinical Studies for Tyrosine Kinase Inhibitors Based on Pharmacokinetics/Pharmacodynamics and Toxicokinetics/Toxicodynamics.https://www.sciencedirect.com/science/article/abs/pii/S1347436715306443
- Principles of dose-setting in toxicology studies: the importance of kinetics for ensuring human safety.https://link.springer.com/article/10.1007/s00204-021-03155-4

