
Introduction
A virtual bioanalytical strategy is no longer optional for early drug discovery—it is a necessity for biotech companies operating with limited capital, lean teams, and aggressive timelines. In the earliest stages of discovery, access to reliable bioanalytical data determines which compounds advance and which fail. Yet, building and maintaining internal bioanalytical infrastructure is costly, slow, and operationally complex.
For early-stage biotechs, adopting a virtual bioanalytical strategy allows scientific teams to focus on innovation while leveraging expert-led bioanalytical services in drug development. When implemented correctly, this approach delivers speed, data integrity, regulatory readiness, and long-term scalability—especially when supported by an experienced bioanalytical CRO for drug discovery.
At ResolveMass Laboratories Inc., we work closely with discovery-stage teams to design virtual bioanalytical frameworks that support confident decision-making from hit identification through lead optimization.
Summary
- A virtual bioanalytical strategy enables early drug discovery teams to access high-quality bioanalysis without building in-house labs.
- Virtual approaches reduce cost, accelerate timelines, and improve scalability for seed-stage and early biotech companies.
- Strategic CRO partnerships, robust data governance, and regulatory-ready workflows are the foundation of success.
- ResolveMass Laboratories Inc. provides expert-driven bioanalytical support tailored for virtual discovery models.
- Implementing the right virtual bioanalytical strategy early minimizes downstream risk and regulatory rework.
1: What Is a Virtual Bioanalytical Strategy?
A bioanalytical strategy is a structured operating model in which bioanalytical activities—such as bioanalytical method development, validation, sample analysis, and data reporting—are conducted through qualified external laboratories rather than in-house facilities.
Rather than simply outsourcing testing, a virtual bioanalytical strategy creates a coordinated, scientifically governed framework that integrates external bioanalysis into the core discovery workflow, often supported by bioanalytical outsourcing models.
This approach enables biotech companies to maintain full scientific ownership while benefiting from specialized platforms such as LC-MS/MS bioanalytical services and ligand-binding assays for both small and large molecule bioanalysis.
In practical terms, it allows drug discovery organizations to operate “virtually” while maintaining full scientific ownership, regulatory awareness, and data traceability.
Core Components of a Virtual Bioanalytical Strategy
A robust virtual bioanalytical strategy is built on several interconnected components:
- Outsourced bioanalytical method development and validation
- High-throughput LC-MS/MS bioanalysis of xenobiotics
- Biomarker bioanalytical services for discovery and translational research
- PK/PD and toxicokinetic bioanalysis
- Audit-ready data systems and regulated bioanalytical services
Each component ensures that bioanalysis supports—not limits—the pace and quality of early drug discovery.
2: Why Early Drug Discovery Teams Need a Virtual Bioanalytical Strategy
Early discovery programs face unique scientific and operational challenges that make a bioanalytical strategy particularly advantageous.
Key Challenges in Early Drug Discovery
- Limited funding and runway → addressed through affordable bioanalytical services for biotech startups
- Small internal teams → supported by bioanalytical CRO services for PK and TK
- Rapid compound iteration → enabled by high-throughput bioanalysis
- Evolving target biology and assay requirements
- Uncertain regulatory paths → mitigated by discovery vs regulated bioanalysis alignment
- Pressure to generate decision-quality data quickly
A well-designed bioanalytical strategy directly addresses these challenges by eliminating fixed infrastructure costs while preserving analytical rigor, flexibility, and regulatory awareness.
3: How a Virtual Bioanalytical Strategy Reduces Cost and Risk
A bioanalytical strategy converts capital expenditure into predictable operational costs, helping biotech teams manage bioanalytical testing services cost.
Instead of committing capital to facilities, equipment, and long-term staffing, organizations convert bioanalysis into predictable, milestone-based operational expenses.
Cost Comparison: In-House vs Virtual Bioanalytical Strategy
| Aspect | In-House Bioanalysis | Virtual Bioanalytical Strategy |
|---|---|---|
| Capital investment | Very high | Minimal |
| Setup time | 12–24 months | Immediate |
| Staffing | Full-time hires | On-demand experts |
| Scalability | Limited | Highly flexible |
| Regulatory readiness | Variable | CRO-driven |
This approach allows early-stage programs to preserve capital for medicinal chemistry, biology, and translational research—while still generating robust pharmacokinetic (PK), toxicokinetic (TK), and biomarker data.
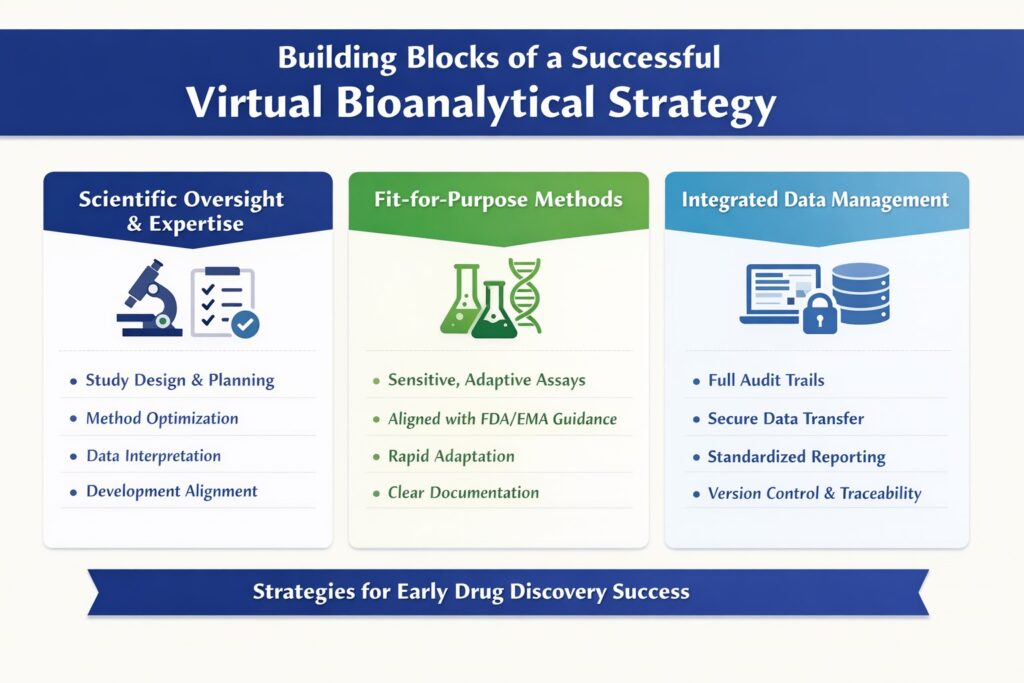
4: Building Blocks of a Successful Virtual Bioanalytical Strategy
1. Scientific Oversight and Expertise
A bioanalytical strategy must be guided by experts who understand assay science, regulatory expectations, and the uncertainties inherent in early discovery. Without strong scientific leadership, outsourced bioanalysis risks becoming transactional rather than strategic.
Strong oversight ensures bioanalysis remains strategic rather than transactional. ResolveMass provides senior-level involvement across bioanalytical laboratory services and regulated workflows.
At ResolveMass Laboratories Inc., senior bioanalytical scientists remain directly involved in:
- Study design and analytical planning
- Method selection and optimization
- Data interpretation and troubleshooting
- Alignment of discovery data with future development needs
This level of engagement ensures scientific continuity and accountability.
2. Fit-for-Purpose Bioanalytical Methods
In early discovery, bioanalytical methods should be fit-for-purpose, not over-engineered or over-validated.
Discovery-stage methods must be scientifically justified and flexible, accounting for challenges such as bioanalytical matrix effects and evolving chemistry.
A strong virtual bioanalytical strategy ensures that methods are:
- Sensitive enough for early PK and exposure studies
- Rapidly adaptable to evolving compound chemistry
- Scientifically justified and clearly documented
- Aligned with FDA and EMA bioanalytical guidance
This approach accelerates discovery while laying a solid foundation for later-stage validation.
3. Integrated Data Management and Traceability
Data integrity is a cornerstone of any virtual bioanalytical strategy. Early discovery data often informs investor decisions, partner discussions, and regulatory planning.
Discovery data often informs IND strategy, making alignment with bioanalytical services for IND and NDA submissions essential.
Key data governance principles include:
- Complete audit trails for all analytical activities
- Secure electronic data transfer and storage
- Standardized reporting formats
- Clear version control and metadata documentation
These practices ensure data reliability, transparency, and long-term usability.
5: Role of CRO Partnerships in a Virtual Bioanalytical Strategy
A bioanalytical strategy succeeds when CROs act as scientific partners. ResolveMass operates as a trusted bioanalytical CRO for virtual biotech and bioanalytical CRO in North America.
What to Look for in a Bioanalytical CRO
- Demonstrated experience in early drug discovery programs
- Ability to scale from discovery to IND-enabling work
- Regulatory-aligned quality systems
- Transparent communication and realistic timelines
- Scientific engagement beyond routine execution
ResolveMass Laboratories Inc. specializes in supporting virtual biotech and discovery-stage teams through customized, science-driven bioanalytical services.
6: Virtual Bioanalytical Strategy Across Discovery Stages
A well-designed bioanalytical strategy evolves alongside the drug discovery lifecycle.
Discovery Phase Alignment
- Hit-to-lead: Exposure screening using bioanalytical quantification
- Lead optimization: PK, metabolism, and LC-MS for large molecules
- Candidate selection: Reproducibility across small vs large molecule bioanalysis
Transition to Preclinical Development
By designing discovery bioanalysis with future requirements in mind, a bioanalytical strategy minimizes method redevelopment during GLP transitions and accelerates program progression.
7: Regulatory Readiness and Compliance Considerations
Even at the discovery stage, regulatory expectations should inform the virtual bioanalytical strategy.
Best Practices for Regulatory-Aligned Bioanalysis
- Follow FDA and EMA bioanalytical method validation guidance
- Clearly document deviations and scientific rationale
- Maintain traceable raw data, calculations, and reports
- Design early methods with future validation pathways in mind
Early alignment with GLP bioanalytical services and clinical bioanalytical services reduces downstream delays.
8: Common Pitfalls in Virtual Bioanalytical Strategy Implementation
Common failures include fragmented vendors, lack of scientific ownership, and underestimating challenges in bioanalytical method development.
Avoid These Common Mistakes
- Selecting CROs based solely on cost
- Lack of internal scientific ownership
- Over- or under-validating analytical methods
- Poor communication and unclear expectations
- Fragmented data across multiple vendors
Partnering with an experienced, discovery-focused bioanalytical team significantly reduces these risks.
9: Why ResolveMass Laboratories Inc. for Virtual Bioanalytical Strategy
ResolveMass Laboratories Inc. brings deep expertise in supporting early-stage and virtual biotech companies throughout the discovery continuum.
What Sets ResolveMass Apart
- Discovery-focused bioanalytical expertise
- Flexible, virtual-friendly engagement models
- Regulatory-ready quality systems
- Strong emphasis on data integrity and traceability
- Collaborative, scientist-to-scientist communication
Our approach ensures your bioanalytical strategy is not simply outsourced—but strategically engineered for long-term success.
ResolveMass offers a complete ecosystem of bioanalytical services supported by deep expertise in:
- Large molecule bioanalysis
- Cell and gene therapy bioanalysis
- Biosimilar bioanalysis
- End-to-end bioanalytical services overview
Conclusion
A thoughtfully designed virtual bioanalytical strategy enables early drug discovery teams to move faster, control costs, and generate decision-quality data. Supported by expert-led bioanalytical CRO services and cost-effective bioanalytical services, virtual bioanalysis becomes a strategic advantage.
If you are building or refining your bioanalytical strategy, the experts at ResolveMass Laboratories Inc. are ready to support your journey.
Frequently Asked Questions:
A virtual bioanalytical strategy is an approach where bioanalytical activities such as method development, validation, and sample analysis are performed by external expert laboratories instead of in-house teams. It allows early drug discovery companies to generate high-quality bioanalytical data without investing in costly infrastructure.
Early-stage biotech companies often operate with limited funding and small teams. A virtual bioanalytical strategy reduces capital expenditure, accelerates timelines, and provides access to specialized expertise, enabling faster and more confident go/no-go decisions during discovery.
A virtual bioanalytical strategy converts fixed laboratory and staffing costs into flexible, milestone-based expenses. Companies avoid long setup times, expensive equipment purchases, and full-time hires while still maintaining regulatory-aligned bioanalytical quality.
A virtual bioanalytical strategy commonly includes bioanalytical method development and validation, LC-MS/MS analysis, PK/PD and toxicokinetic bioanalysis, biomarker assays, and data reporting. These services are tailored to discovery-stage requirements and can scale as programs mature.
Yes, when designed correctly. A well-structured virtual bioanalytical strategy follows FDA and EMA bioanalytical guidance, maintains data integrity, and supports seamless transition into GLP and IND-enabling studies, reducing regulatory risk later.
Traditional outsourcing is often transactional and study-specific. A virtual bioanalytical strategy is a long-term, integrated approach where the CRO acts as a scientific partner, aligning bioanalysis with discovery goals, timelines, and future regulatory needs.
CROs provide scientific expertise, validated platforms, quality systems, and regulatory experience. In a virtual bioanalytical strategy, CROs function as extensions of the internal team, supporting discovery through IND preparation with continuity and accountability.
Reference
- Aarzoo Thakur, Zhiyuan Tan, Tsubasa Kameyama, Eman El-Khateeb, Shakti Nagpal, Stephanie Malone.Bioanalytical strategies in drug discovery and development.https://www.tandfonline.com/doi/abs/10.1080/03602532.2021.1959606
- Saurabh Pandey , Preeti Pandey , Gaurav Tiwari , Ruchi Tiwari.Bioanalysis in drug discovery and development.https://www.sciencedirect.com/science/article/abs/pii/S2229470810110036
- Bioanalysis for Plasma Protein Binding Studies in Drug Discovery and Drug Development: Views and Recommendations of The European Bioanalysis Forum.https://www.tandfonline.com/doi/full/10.4155/bio.13.338
- Mohammad Mahdi Moein.Bioanalytical method development and validation: Critical concepts and strategies.https://www.sciencedirect.com/science/article/abs/pii/S1570023216308881
- Voon S. Ong , Kevin L. Cook , Christine M. Kosara , William F. Brubaker. Quantitative bioanalysis: an integrated approach for drug discovery and development.https://www.sciencedirect.com/science/article/abs/pii/S1387380604003379

