Introduction to GC-MS Testing for Medical Devices
GC-MS testing for medical devices is the most powerful analytical technique for detecting organic extractables and leachables that could pose safety risks to patients. Gas chromatography-mass spectrometry combines superior separation capabilities with definitive identification of volatile and semi-volatile compounds, making it indispensable for medical device biocompatibility assessment.
Medical devices contain various materials—polymers, adhesives, lubricants, sterilants, and additives—that can release chemical substances when in contact with biological fluids or tissues. These released substances, known as extractables and leachables, may cause adverse biological responses ranging from mild irritation to serious systemic toxicity. Understanding and controlling these chemicals through rigorous GC-MS testing for medical devices is fundamental to ensuring patient safety and achieving regulatory approval.
- For readers seeking a deeper technical foundation, a detailed overview of Gas Chromatography–Mass Spectrometry is available here:
👉 https://resolvemass.ca/gas-chromatography-mass-spectrometry/ - An explanation of the working principle of GC-MS can also be explored at:
👉 https://resolvemass.ca/working-principle-of-gc-ms/
At ResolveMass Laboratories Inc., we recognize that medical device manufacturers face increasingly stringent regulatory scrutiny regarding extractables and leachables. Our specialized GC-MS testing for medical devices provides the comprehensive chemical characterization required by global regulatory authorities including the FDA, Health Canada, and European Medicines Agency. This article explains the critical role of GC-MS testing in medical device safety assessment and how proper E&L evaluation protects patients while supporting successful regulatory submissions.
Summary
GC-MS testing for medical devices is essential for identifying and quantifying extractables and leachables (E&L) that could compromise patient safety. This comprehensive guide covers:
- Extractables Testing: GC-MS identifies potential chemical compounds that can be extracted from medical device materials under controlled laboratory conditions using aggressive solvents
- Leachables Testing: Real-world simulation testing detects chemicals that migrate from devices during normal use, storage, or sterilization
- Regulatory Requirements: ISO 10993-18, FDA guidance, and USP standards mandate comprehensive E&L assessment for all patient-contacting medical devices
- Risk Assessment: Systematic evaluation of toxicological hazards from identified extractables and leachables ensures patient safety
- Testing Protocols: Customized GC-MS methods based on device type, contact duration, and patient exposure routes deliver reliable safety data
A broader overview of GC-MS applications across regulated industries can be found here:
👉 https://resolvemass.ca/applications-of-gcms/
1: Understanding Extractables and Leachables in Medical Devices
Extractables are chemical compounds released from medical device materials under aggressive laboratory conditions, while leachables are substances that migrate from devices under normal use conditions. This distinction is critical for proper safety assessment.
Defining Extractables
Extractables represent the “worst-case” chemical profile of a medical device material. Key characteristics include:
- Extraction conditions: Aggressive solvents (water, alcohol, hexane, acidic/basic solutions)
- Elevated temperatures: Often 40-70°C to accelerate extraction
- Extended duration: Typically 24-72 hours or longer
- Purpose: Identify all potential chemical migrants for toxicological assessment
Defining Leachables
Leachables represent chemicals that actually migrate during real-world device use:
- Realistic conditions: Actual use media (saline, blood, tissue contact)
- Normal temperatures: Body temperature (37°C) or storage conditions
- Actual duration: Matches intended use timeframe
- Purpose: Confirm which extractables actually appear during clinical use
Why Both Extractables and Leachables Testing Matter
| Testing Type | Purpose | Regulatory Stage | GC-MS Application |
|---|---|---|---|
| Extractables | Comprehensive chemical profiling | Early development, material selection | Identifies full range of potential migrants |
| Leachables | Real-world safety confirmation | Final device testing, clinical studies | Confirms actual patient exposure levels |
2: Regulatory Framework for GC-MS Testing for Medical Devices
ISO 10993-18:2020 provides the international standard for extractables and leachables assessment in medical devices, requiring systematic chemical characterization using techniques like GC-MS. Compliance with this standard is essential for global market access.
Key Regulatory Guidelines
- ISO 10993-18:2020 – Biological Evaluation of Medical Devices
- Part 18: Chemical characterization of medical device materials
- Mandates extractables and leachables studies for all patient-contacting devices
- Specifies analytical methods including GC-MS testing for medical devices
- FDA Guidance Documents
- “Use of International Standard ISO 10993-1” (2020)
- Chemistry recommendations for device evaluation
- Biocompatibility assessment framework
- USP <661> and <1664>
- Plastic materials of construction
- Assessment of extractables from plastic delivery systems
- Analytical method requirements
- European Medical Device Regulation (MDR 2017/745)
- Chemical safety requirements
- Technical documentation expectations
- Clinical evaluation integration
Contact Duration Categories and Testing Requirements
The extent of GC-MS testing for medical devices depends on patient contact duration:
| Contact Category | Duration | Examples | E&L Testing Intensity |
|---|---|---|---|
| Limited | ≤24 hours | Surgical instruments, catheters | Moderate extractables screening |
| Prolonged | 24 hours – 30 days | Implants, drug delivery devices | Comprehensive E&L assessment |
| Permanent | >30 days | Cardiac implants, orthopedic devices | Extensive extraction and leaching studies |
3: GC-MS Testing Methodology for Medical Device Extractables
GC-MS testing for medical devices during extractables assessment uses controlled laboratory extractions followed by comprehensive chemical analysis to identify all potential migrants. This systematic approach ensures complete material characterization.
Sample Preparation for Extractables Testing
Our extractables testing protocol follows ISO 10993-18 recommendations:
- Material Selection
- Representative samples from final manufactured device
- Worst-case components with maximum patient contact
- Surface area calculation for proper extraction ratios
- Solvent Selection
- Polar solvents: Water, ethanol (simulating aqueous biological fluids)
- Non-polar solvents: Hexane, vegetable oil (simulating lipophilic contact)
- pH-adjusted solutions: Acidic and basic conditions (pH 3 and 10)
- Extraction Conditions
- Temperature: Typically 50°C or 70°C for accelerated extraction
- Duration: 24-72 hours depending on device contact category
- Sample-to-solvent ratio: Based on clinical use surface area
GC-MS Analysis Parameters for Extractables
Our GC-MS testing for medical devices employs optimized analytical parameters to detect volatile and semi-volatile organic compounds. Key method specifications include:
- Column selection: Non-polar (DB-5, HP-5) for broad compound coverage
- Temperature programming: Typically 40-300°C with appropriate ramp rates
- Injection techniques: Split/splitless injection, headspace for volatiles
- Mass spectrometry: Full scan mode (m/z 35-550) for unknown identification
- Detection limits: Parts-per-million (ppm) to parts-per-billion (ppb) levels
Compound Identification and Reporting
ResolveMass Laboratories Inc. provides comprehensive extractables profiles including:
- Spectral library matching: NIST, Wiley, custom medical device databases
- Confirmation criteria: Match quality >800, retention index verification
- Quantification: Semi-quantitative or absolute using appropriate standards
- Chemical classification: Grouping by compound class (plasticizers, antioxidants, monomers)
For laboratories supporting regulated analytical workflows, explore GC-MS analysis services here:
👉 https://resolvemass.ca/gcms-analysis-service/
👉 https://resolvemass.ca/gcms-analysis-service-2/
4: GC-MS Testing for Leachables in Medical Devices
Leachables testing using GC-MS for medical devices simulates actual use conditions to determine which extractables migrate during clinical application. This testing provides direct patient exposure data critical for safety assessment.
Leachables Study Design Principles
Effective leachables testing requires careful experimental design:
- Use-Simulation Selection
- Match clinical use media (saline, blood, tissue simulants)
- Replicate actual contact temperature and duration
- Include worst-case scenarios (extended storage, multiple use cycles)
- Device Configuration
- Test complete device as clinically used
- Include all patient-contacting components
- Account for sterilization and packaging effects
- Time Points
- Initial leaching (first contact)
- Throughout use duration
- End-of-life or expiry conditions
Specialized GC-MS Approaches for Leachables
GC-MS testing for medical devices during leachables assessment often requires specialized techniques to handle complex biological matrices. Our laboratory employs:
- Headspace GC-MS: For volatile compounds in aqueous leachate
- Thermal desorption GC-MS: Direct analysis of device surfaces
- Derivatization techniques: For polar, non-volatile compounds
- High-resolution MS: Accurate mass determination for unknown identification
Leachables Threshold Concepts
Regulatory guidance establishes analytical thresholds for leachables reporting:
| Threshold Type | Typical Level | Purpose |
|---|---|---|
| Analytical Evaluation Threshold (AET) | 0.15 μg/day per component | Screening level for identification and reporting |
| Safety Concern Threshold (SCT) | 1.5 μg/day | Requires toxicological assessment |
| Qualification Threshold | Device-specific | Based on risk assessment and contact duration |
5: Comprehensive E&L Testing Program Design
A complete extractables and leachables program using GC-MS testing for medical devices follows a systematic workflow from material characterization through risk assessment. This structured approach ensures regulatory compliance and patient safety.
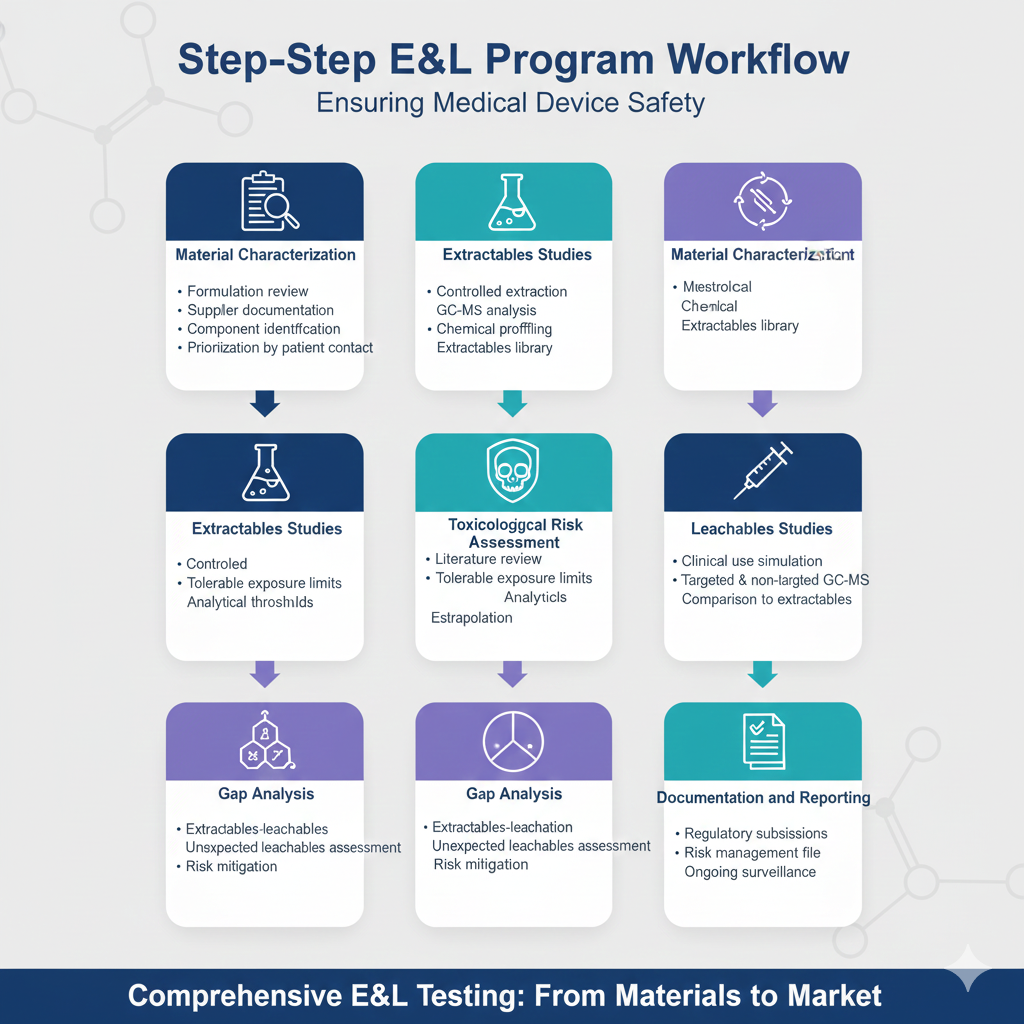
Step-by-Step E&L Program Workflow
- Material Characterization
- Formulation review and supplier documentation
- Identification of all device components
- Prioritization based on patient contact
- Extractables Studies
- Controlled extraction under multiple conditions
- GC-MS analysis with comprehensive chemical profiling
- Creation of extractables library
- Toxicological Risk Assessment
- Literature review for identified extractables
- Calculation of tolerable exposure limits
- Establishment of analytical thresholds
- Leachables Studies
- Clinical use simulation testing
- Targeted and non-targeted GC-MS analysis
- Comparison to extractables profile
- Gap Analysis
- Correlation of extractables to leachables
- Assessment of unexpected leachables
- Risk characterization and mitigation
- Documentation and Reporting
- Regulatory submission packages
- Risk management file integration
- Ongoing surveillance protocols
Custom method development is often required, particularly for novel polymers or complex assemblies. Learn more about GC-MS method development services:
👉 https://resolvemass.ca/gcms-method-development-service/
👉 https://resolvemass.ca/gc-ms-method-development/
6: Common Chemical Classes Detected by GC-MS in Medical Devices
GC-MS testing for medical devices routinely identifies specific chemical classes that present potential safety concerns. Understanding these compound categories helps manufacturers make informed material selection decisions.
For insight into trace-level solvent and volatile impurity analysis, see:
👉 https://resolvemass.ca/gcms-residual-solvent-analysis-what-you-must-know/
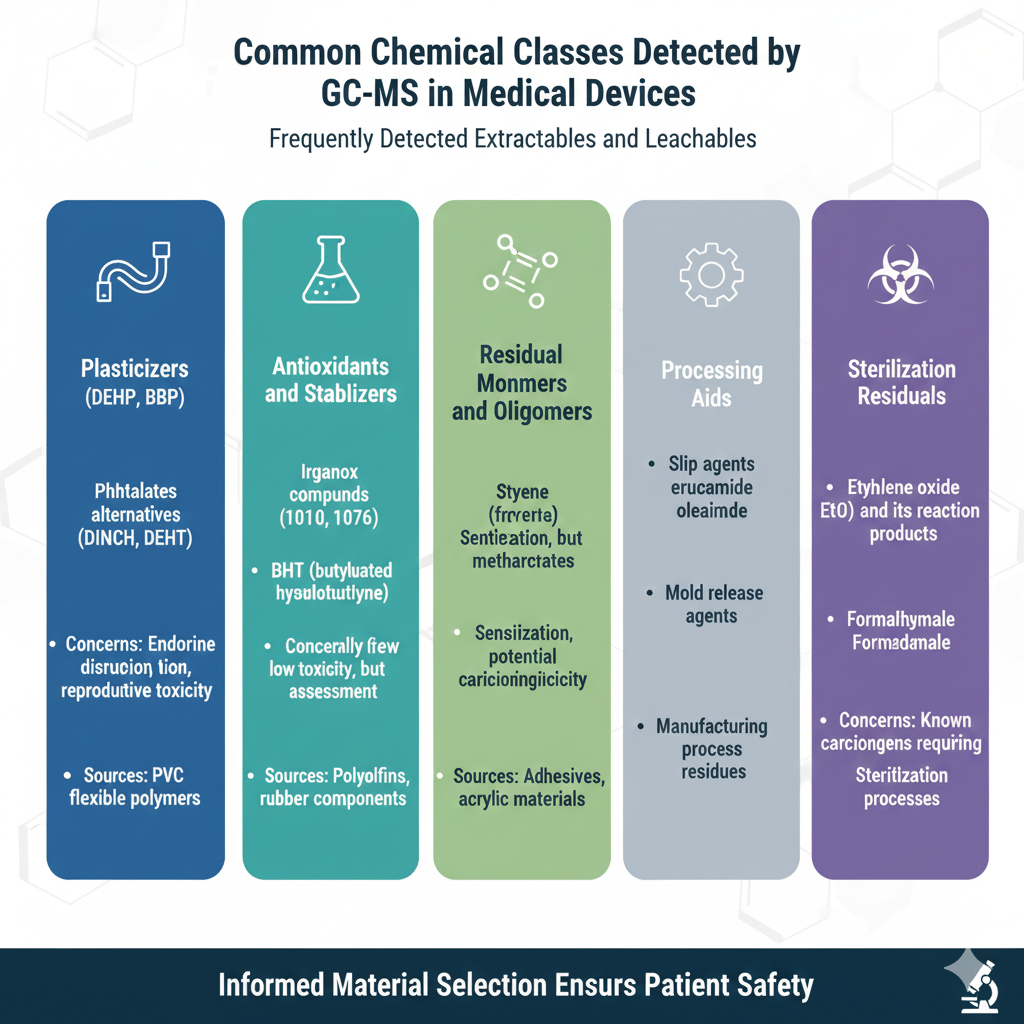
Frequently Detected Extractables and Leachables
Plasticizers
- Phthalates (DEHP, DBP, BBP)
- Non-phthalate alternatives (DINCH, DEHT)
- Concerns: Endocrine disruption, reproductive toxicity
- Sources: PVC tubing, flexible polymers
Antioxidants and Stabilizers
- Irganox compounds (1010, 1076)
- BHT (butylated hydroxytoluene)
- Concerns: Generally low toxicity, but require assessment
- Sources: Polyolefins, rubber components
Residual Monomers and Oligomers
- Styrene (from polystyrene)
- Acrylates and methacrylates
- Concerns: Sensitization, potential carcinogenicity
- Sources: Adhesives, acrylic materials
Processing Aids
- Slip agents (erucamide, oleamide)
- Mold release agents
- Concerns: Generally low toxicity
- Sources: Manufacturing process residues
Sterilization Residuals
- Ethylene oxide (EtO) and its reaction products
- Formaldehyde
- Concerns: Known carcinogens requiring strict limits
- Sources: Sterilization processes
7: Advantages of GC-MS Over Alternative Analytical Techniques
GC-MS testing for medical devices offers distinct advantages over other analytical methods for extractables and leachables assessment. While multiple techniques may be required for comprehensive characterization, GC-MS provides unique capabilities.
Comparative Analytical Techniques
| Technique | Best For | Limitations | GC-MS Advantage |
|---|---|---|---|
| GC-MS | Volatile and semi-volatile organics | Non-volatile compounds | Structural identification, quantification |
| LC-MS | Polar, non-volatile compounds | Volatile compounds | GC-MS complements for complete coverage |
| ICP-MS | Elemental analysis (metals) | Organic compounds | GC-MS handles organic extractables |
| IC | Ionic species | Organic molecules | GC-MS provides molecular information |
| FTIR | Functional group screening | Not compound-specific | GC-MS gives definitive identification |
Why GC-MS Remains Essential
Our GC-MS testing for medical devices is irreplaceable because it:
- Identifies unknowns: Mass spectral libraries enable identification without reference standards
- Quantifies trace levels: Sub-ppm sensitivity for safety-critical compounds
- Separates complex mixtures: Chromatographic resolution of multi-component extracts
- Provides structural information: Fragmentation patterns reveal molecular structure
- Offers universal detection: Responds to virtually all organic compounds
8: ResolveMass Laboratories Inc. Medical Device Testing Capabilities
ResolveMass Laboratories Inc. delivers comprehensive GC-MS testing for medical devices with specialized expertise in extractables and leachables assessment. Our laboratory serves as a trusted partner for medical device manufacturers worldwide.
Our Specialized Service Portfolio
Extractables Testing Services
- Complete material characterization programs
- Multi-solvent extraction protocols per ISO 10993-18
- Comprehensive chemical profiling with GC-MS and complementary techniques
- Extractables library development for device families
Leachables Testing Services
- Clinical use simulation studies
- Real-time and accelerated leaching protocols
- Targeted analysis for known extractables
- Non-targeted screening for unexpected migrants
Method Development and Validation
- Custom GC-MS methods for specific device types
- Validation per ICH Q2(R1) principles adapted for medical devices
- Technology transfer and method implementation support
- Analytical troubleshooting and optimization
Regulatory Support Services
- ISO 10993-18 gap analysis and strategy development
- Toxicological risk assessment support
- Regulatory submission documentation preparation
- Pre-submission meetings and agency interaction support
Quality Systems and Accreditations
Our GC-MS testing for medical devices operates under stringent quality standards:
- ISO/IEC 17025 Accreditation: Internationally recognized testing competence
- ISO 13485 Compliance: Medical device quality management system
- FDA-Inspected Facility: Proven regulatory readiness
- 21 CFR Part 11: Electronic records and signatures compliance
- GLP Compliance: Good Laboratory Practice for safety studies
Advanced Instrumentation Suite
ResolveMass Laboratories Inc. maintains state-of-the-art GC-MS technology:
- High-resolution GC-MS: Accurate mass determination for unknown identification
- GC-MS/MS: Enhanced sensitivity for trace-level leachables
- Headspace GC-MS: Automated volatile compound analysis
- Thermal desorption GC-MS: Direct surface analysis capabilities
- Pyrolysis GC-MS: Polymer composition and additive screening
Our experience spans multiple regulated industries, including:
- Pharmaceutical GC-MS analysis
👉 https://resolvemass.ca/gc-ms-analysis-for-pharmaceuticals/ - GC-MS analysis in the United States
👉 https://resolvemass.ca/gcms-analysis-in-united-states/ - GC-MS analysis in Montreal, Canada
👉 https://resolvemass.ca/gcms-analysis-in-montreal/
👉 https://resolvemass.ca/gcms-analysis-in-montreal-canada-why-resolvemass-laboratories-inc-is-your-best-choice/
We also support complex matrices such as:
- Plant extract analysis using GC-MS
👉 https://resolvemass.ca/gcms-analysis-of-plant-extract/ - Pesticide testing using GC-MS in Canada
👉 https://resolvemass.ca/pesticide-testing-services-using-gc-ms-in-canada/
9: Common Medical Device Types Requiring GC-MS Testing
Virtually all patient-contacting medical devices require GC-MS testing for extractables and leachables as part of biocompatibility assessment. Testing protocols vary based on device characteristics and risk profile.
A comparative guide for evaluating laboratories is available here:
👉 https://resolvemass.ca/best-gc-ms-analysis-services-in-north-america-how-to-compare-labs-for-accuracy-and-turnaround-time/
Device Categories and E&L Considerations
Cardiovascular Devices
- Catheters, guidewires, stents, heart valves
- Direct blood contact requires comprehensive E&L assessment
- Particular concern for thrombogenic or hemolytic compounds
Orthopedic Implants
- Joint replacements, bone plates, spinal implants
- Permanent contact necessitates extensive testing
- Focus on polymer components (UHMWPE, PEEK)
Drug Delivery Systems
- Prefilled syringes, autoinjectors, infusion sets
- E&L may impact drug product quality and patient safety
- Interaction studies between device and pharmaceutical required
Respiratory Devices
- Breathing circuits, masks, ventilator components
- Inhaled exposure pathway increases toxicological concern
- Volatile compounds of particular interest
Wound Care and Surgical Products
- Dressings, surgical meshes, sutures
- Extended tissue contact requires thorough evaluation
- Adhesive components often present E&L challenges
10: Toxicological Risk Assessment for Identified Compounds
After GC-MS testing for medical devices identifies extractables and leachables, toxicological evaluation determines whether exposure levels present acceptable patient risk. This assessment integrates analytical data with safety science.
Risk Assessment Framework
The toxicological evaluation process follows these steps:
- Compound Identification
- Definitive identification via GC-MS analysis
- CAS number assignment for literature searching
- Exposure Calculation
- Daily dose based on leachables concentration
- Consideration of contact duration and body weight
- Route-specific adjustments (dermal, inhalation, blood contact)
- Hazard Assessment
- Literature review for toxicological data
- Consultation of regulatory databases (ECHA, EPA, FDA)
- Classification by toxicity endpoints
- Risk Characterization
- Comparison of exposure to tolerable limits (TTC, ADE, SCT)
- Application of safety factors
- Risk acceptability determination
Safety Thresholds and Decision Trees
| Exposure Level | Action Required |
|---|---|
| Below AET | Identification not required, monitor in ongoing testing |
| AET to SCT | Identify, report, generally acceptable based on TTC principles |
| Above SCT | Identify, full toxicological assessment required, may need mitigation |
| Above established limits | Material substitution or process changes mandatory |
11: Case Studies: GC-MS Testing Resolving Device Safety Challenges
Our GC-MS testing for medical devices has helped numerous manufacturers identify and resolve extractables and leachables issues before market release. While maintaining client confidentiality, representative examples illustrate our problem-solving capabilities.
Catheter Leachables Investigation
Challenge: Unexpected adverse events during clinical trials suggested chemical exposure Approach: Comprehensive GC-MS testing for medical devices including targeted leachables analysis Finding: Previously unidentified plasticizer migration above safety threshold Resolution: Material reformulation and validated alternative material selection
Implant Material Qualification
Challenge: New polymer blend required complete E&L characterization for regulatory submission Approach: Multi-solvent extractables study with GC-MS and complementary LC-MS Finding: Complete chemical profile with all compounds below safety concern levels Resolution: Successful regulatory approval with comprehensive E&L documentation
Sterilization Process Optimization
Challenge: Ethylene oxide residuals exceeding FDA limits after standard sterilization Approach: GC-MS method development for EtO and reaction products with enhanced sensitivity Finding: Detailed characterization of EtO distribution and off-gassing kinetics Resolution: Optimized aeration protocol ensuring compliance with residual limits
12: Best Practices for Medical Device E&L Programs
Successful implementation of GC-MS testing for medical devices requires strategic planning and adherence to scientific best practices. These principles guide effective E&L programs.
Early-Stage Recommendations
- Material selection: Choose materials with established biocompatibility history
- Supplier engagement: Obtain comprehensive formulation information early
- Extractables screening: Conduct preliminary testing during development
- Design consideration: Minimize patient contact with highest-risk materials
Testing Strategy Optimization
- Phased approach: Screen with extractables, confirm with leachables
- Complementary techniques: Combine GC-MS with LC-MS for complete coverage
- Appropriate controls: Include positive and negative control materials
- Repeatability: Multiple extraction replicates ensure reliable data
Regulatory Documentation
- Comprehensive reporting: Include all identified compounds above AET
- Risk justification: Clear rationale for safety conclusions
- Method validation: Document analytical method performance characteristics
- Change control: Plan for material changes requiring re-testing
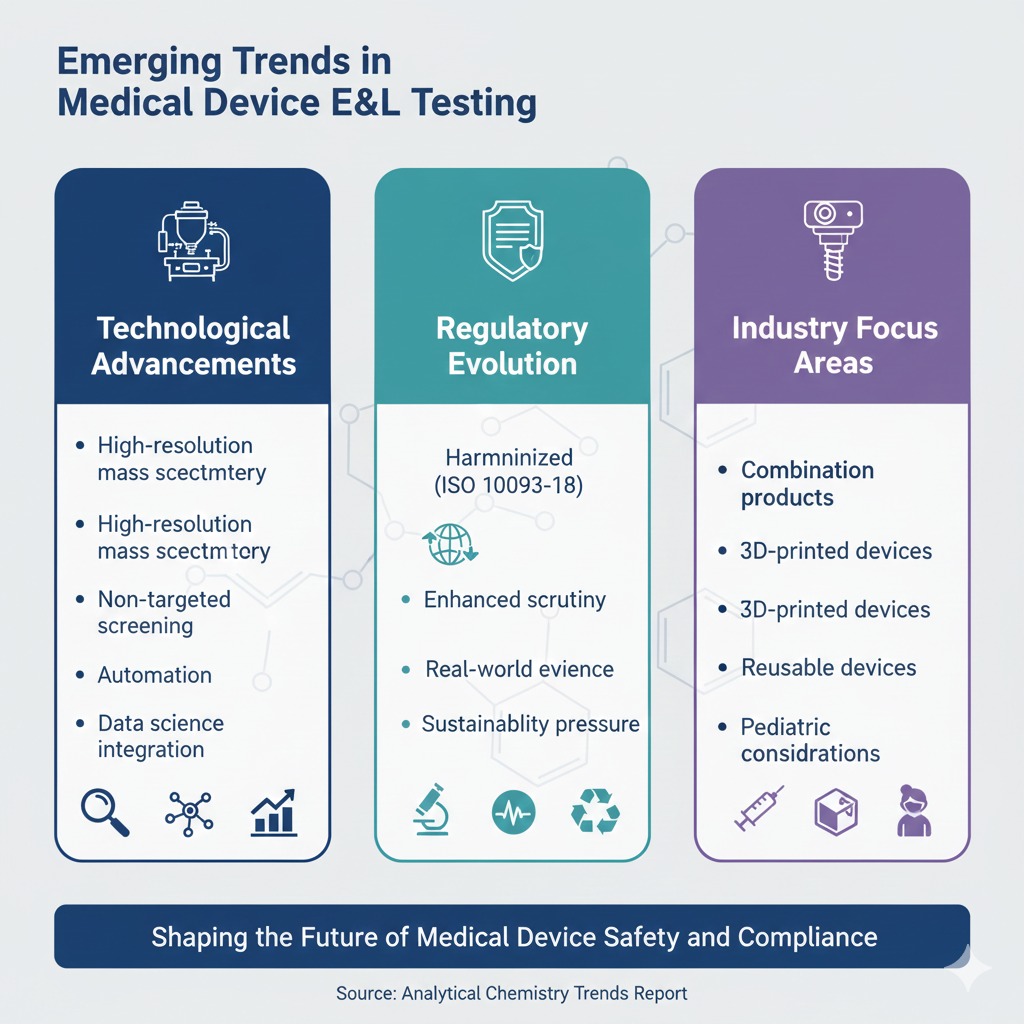
13: Emerging Trends in Medical Device E&L Testing
The field of GC-MS testing for medical devices continues evolving with advancing technology and changing regulatory expectations. Key trends shaping the future include:
Technological Advancements
- High-resolution mass spectrometry: Improved unknown identification with accurate mass
- Non-targeted screening: Advanced data analysis for unexpected compounds
- Automation: High-throughput sample preparation and analysis
- Data science integration: Machine learning for compound prediction and risk assessment
Regulatory Evolution
- Harmonized standards: Global alignment around ISO 10993-18 principles
- Enhanced scrutiny: Increased regulatory focus on polymer additives and processing aids
- Real-world evidence: Post-market surveillance for leachables-related adverse events
- Sustainability pressure: Evaluation of bio-based and recycled materials
Industry Focus Areas
- Combination products: Complex E&L interactions between device and drug
- 3D-printed devices: Novel materials requiring comprehensive characterization
- Reusable devices: Long-term leaching assessment for multiple-use medical devices
- Pediatric considerations: Lower exposure thresholds for vulnerable populations
Conclusion
GC-MS testing for medical devices represents the analytical cornerstone of extractables and leachables assessment, ensuring patient safety through comprehensive chemical characterization. As medical device complexity increases and regulatory requirements become more stringent, the importance of rigorous E&L evaluation continues growing.
Medical device manufacturers must approach extractables and leachables testing systematically, beginning with material selection and continuing through post-market surveillance. GC-MS testing for medical devices provides the sensitivity, selectivity, and identification power needed to detect and characterize potential safety concerns before they reach patients. When combined with appropriate toxicological risk assessment, this analytical approach enables informed decision-making that balances innovation with patient protection.
ResolveMass Laboratories Inc. stands as your partner in navigating the complex landscape of medical device E&L testing. Our specialized expertise in GC-MS testing for medical devices, combined with comprehensive regulatory knowledge and advanced analytical capabilities, supports successful product development and regulatory approval. From initial material screening through final leachables confirmation, our team delivers the precise, reliable data your medical device program demands.
Whether you’re developing innovative new devices, qualifying materials for next-generation products, or addressing regulatory questions on existing devices, our GC-MS testing for medical devices provides the analytical foundation for confident safety assessment. We’re committed to advancing medical device safety through scientific excellence and regulatory expertise.
👉 Explore our complete GC-MS Analysis Services here:
https://resolvemass.ca/gcms-analysis-service/
FAQs on GC-MS Testing for Medical Devices
Extractables are chemical compounds that can be released from a medical device when exposed to aggressive solvents, elevated temperatures, or extended contact times during laboratory testing. Leachables are compounds that actually migrate from the device into the drug product, biological fluid, or patient under normal conditions of use. Identifying both is critical to evaluating patient exposure and ensuring device safety.
GC-MS is a key analytical technique for E&L studies because it provides high sensitivity and structural identification for volatile and semi-volatile organic compounds. Many extractables and leachables—such as plasticizers, residual solvents, antioxidants, and degradation products—are ideally suited for GC-MS analysis, making it indispensable in medical device risk assessment.
E&L testing is required for medical devices that come into direct or indirect contact with patients or drug products, including:
-Implantable devices
-Drug-device combination products
-IV sets, syringes, tubing, and catheters
-Inhalation and infusion devices
Regulatory agencies expect E&L evaluation whenever materials may pose a chemical exposure risk.
Extractables and leachables studies are guided by:
-ISO 10993-18 (chemical characterization)
-ISO 10993-17 (toxicological risk assessment)
-FDA guidance for medical devices and combination products
-USP <1663> and <1664> (for packaging and delivery systems)
GC-MS data supports compliance with these global regulatory expectations.
GC-MS commonly detects:
-Residual solvents
-Plasticizers (e.g., phthalates)
-Antioxidants and stabilizers
-Oligomers and low-molecular-weight additives
-Volatile degradation products
These compounds often originate from polymers, adhesives, inks, coatings, and packaging materials.
Extractables studies involve exposing medical device materials to aggressive extraction conditions, such as:
-Multiple solvents (polar and non-polar)
-Elevated temperatures
-Extended extraction times
The goal is to generate a worst-case chemical profile, which is then analyzed by GC-MS to identify and semi-quantify potential migrants.
Reference
- Software tools that enable confident GC-MS analysis of extractables in pharmaceutical products.https://www.europeanpharmaceuticalreview.com/article/78641/gc-ms-analysis-pharmaceuticals/
- Analysis of Extractable/Leachable Compounds from Generic Liquid Drug Formulations Using GC/MSD Systems.https://www.agilent.com/cs/library/applications/5991-5632EN.pdf
- Medical Device Extractables and Leachables Testing.https://www.campoly.com/services/regulatory-testing-services-support/extractables-leachables/medical-device-extractables-and-leachables-testing/

