Introduction
Bioanalytical matrix effects represent one of the most significant challenges in mass spectrometry-based analytical chemistry today. These effects occur when endogenous or exogenous compounds present in biological samples interfere with the ionization process, leading to inaccurate quantification of target analytes. For pharmaceutical companies, contract research organizations, and clinical laboratories, understanding and controlling bioanalytical matrix effects is not just a technical requirement—it’s essential for ensuring patient safety, regulatory compliance, and the integrity of scientific research.
At ResolveMass Laboratories Inc., we’ve dedicated over a decade to perfecting methodologies that identify, quantify, and mitigate matrix interference in complex biological samples. Our expertise in handling these analytical challenges has made us a trusted partner for bioanalytical testing across North America.
At ResolveMass Laboratories Inc., we bring more than a decade of hands-on experience delivering high-quality bioanalytical services in drug development with a strong focus on mitigating matrix interference in LC-MS/MS workflows for small and large molecules.
Article Summary
Key Takeaways:
- Bioanalytical matrix effects occur when components in biological samples interfere with mass spectrometry ionization, causing signal suppression or enhancement
- Matrix effects can reduce analytical accuracy by 20-80% if not properly controlled, compromising drug development and clinical testing
- Ion suppression is the most common type of bioanalytical matrix effects, particularly in electrospray ionization (ESI)
- Proper sample preparation, matrix-matched calibration, and internal standard selection are critical mitigation strategies
- ResolveMass Laboratories employs advanced LC-MS/MS techniques to minimize matrix interference and ensure reliable bioanalytical results
- Regular method validation and post-column infusion tests help identify and quantify matrix effects
- Understanding matrix effects is essential for regulatory compliance in pharmaceutical analysis
1: What Are Bioanalytical Matrix Effects?
Bioanalytical matrix effects are alterations in mass spectrometry signal intensity caused by co-eluting compounds in biological matrices such as plasma, serum, urine, or tissue homogenates. These interferences can either suppress or enhance the ionization efficiency of target analytes, leading to inaccurate quantification.
These effects directly impact the accuracy of bioanalytical quantification, a core service at ResolveMass
Matrix effects primarily manifest in two ways:
- Ion Suppression: Reduction in analyte signal due to competition for ionization or charge neutralization (most common)
- Ion Enhancement: Increase in analyte signal due to improved ionization efficiency in the presence of matrix components
The severity of bioanalytical matrix effects depends on several factors including sample complexity, ionization technique, chromatographic separation, and the chemical properties of both the analyte and matrix components.
These challenges are routinely addressed during bioanalytical method development
2: Understanding Matrix Components in Bioanalytical Samples
Biological matrices contain thousands of endogenous compounds that can interfere with mass spectrometry analysis:
Common Matrix Interferents:
| Matrix Type | Primary Interferents | Impact on Analysis |
|---|---|---|
| Plasma/Serum | Phospholipids, proteins, salts | High ion suppression in ESI |
| Urine | Urea, creatinine, organic acids | Variable pH effects |
| Tissue Homogenates | Lipids, cellular debris, enzymes | Severe signal suppression |
| Whole Blood | Hemoglobin, clotting factors | Complex ionization interference |
These components can co-elute with target analytes during liquid chromatography, entering the ionization source simultaneously and competing for available charge, which directly impacts the reliability of quantitative results.
These challenges are particularly relevant in small- and large-molecule bioanalysis
👉 https://resolvemass.ca/small-molecule-vs-large-molecule-bioanalysis/
👉 https://resolvemass.ca/large-molecule-bioanalysis/
3: How Bioanalytical Matrix Effects Impact Mass Spectrometry Results
Signal Accuracy and Precision
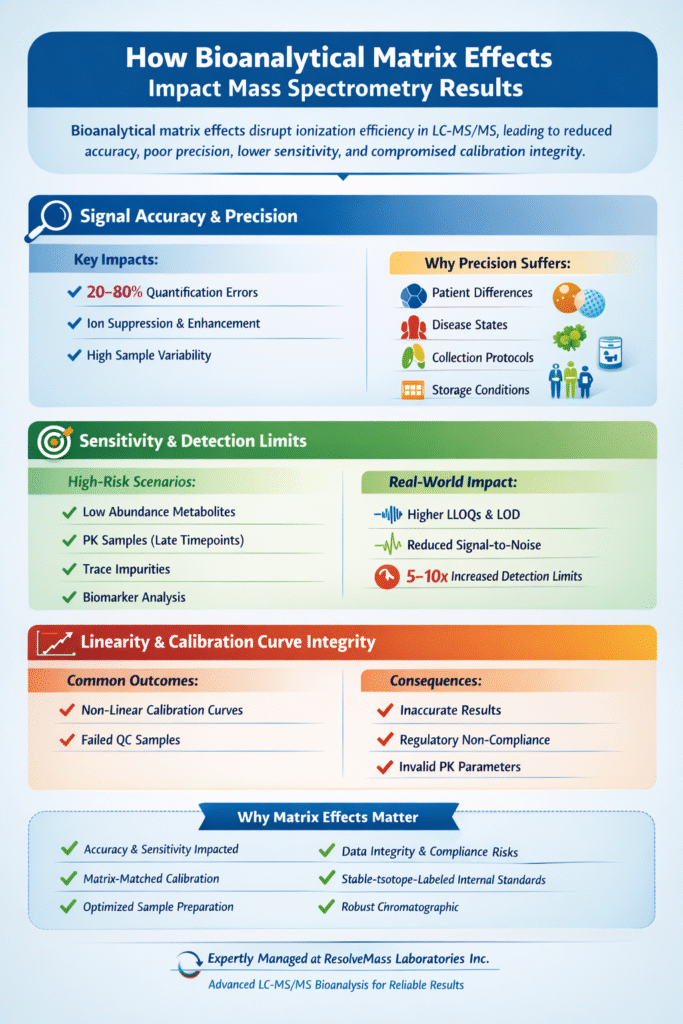
Bioanalytical matrix effects directly compromise measurement accuracy by causing discrepancies between calibration standards prepared in clean solvents and actual biological samples. Studies have shown that uncontrolled matrix effects can introduce quantification errors ranging from 20% to 80%, making them a critical concern in regulated bioanalysis.
Differences between neat standards and real biological samples distort quantitative results, particularly in regulated environments such as PK/PD bioanalysis
The impact on precision is equally concerning. When matrix composition varies between samples—as commonly occurs with different patient populations, disease states, or sample collection protocols—the degree of ion suppression or enhancement changes, leading to poor inter-sample reproducibility.
Sensitivity and Detection Limits
Ion suppression caused by bioanalytical matrix effects reduces the effective sensitivity of mass spectrometry methods. This is particularly problematic when analyzing low-abundance metabolites, trace impurities, or pharmacokinetic samples at late timepoints where drug concentrations are minimal.
Ion suppression caused by bioanalytical matrix effects reduces method sensitivity, especially in late-timepoint pharmacokinetic samples and biomarker bioanalytical services
At ResolveMass Laboratories, we’ve observed that severe matrix effects can elevate method detection limits by 5-10 fold, potentially rendering validated methods unsuitable for their intended purpose.
Linearity and Calibration Curve Integrity
Matrix effects in bioanalytical methods can distort calibration curve linearity, creating non-parallel response between standards and samples. This phenomenon violates fundamental assumptions of quantitative analysis and can lead to:
- Inaccurate back-calculation of analyte concentrations
- Failed quality control samples
- Regulatory non-compliance
- Invalid pharmacokinetic parameters
4: Root Causes of Bioanalytical Matrix Effects in LC-MS/MS
Ionization Source Competition
In electrospray ionization (ESI), the most common technique for bioanalytical applications, matrix components compete with target analytes for available charge during droplet formation and evaporation. Compounds with higher surface activity or gas-phase basicity can preferentially capture protons, suppressing ionization of the analyte of interest.
Electrospray ionization (ESI), widely used in LC-MS/MS bioanalysis of xenobiotics, is highly sensitive to matrix interference
Co-elution of Interferents
Insufficient chromatographic separation allows matrix components to co-elute with target analytes. Phospholipids are notorious offenders in this regard, as their broad structural diversity makes complete chromatographic resolution nearly impossible without specialized techniques.
Poor chromatographic separation allows phospholipids and metabolites to co-elute with analytes—one of the most common challenges in bioanalytical method development
Ionization Technique Sensitivity
Different ionization methods exhibit varying susceptibility to bioanalytical matrix effects:
Ionization Technique Comparison:
- ESI (Electrospray): Most susceptible to matrix effects, especially from phospholipids and salts
- APCI (Atmospheric Pressure Chemical Ionization): More robust against certain matrix effects but limited compound applicability
- APPI (Atmospheric Pressure Photoionization): Intermediate susceptibility, useful for non-polar compounds
- MALDI (Matrix-Assisted Laser Desorption): Different matrix effect profile, tissue imaging applications
5: Strategies to Minimize Bioanalytical Matrix Effects
Sample Preparation Optimization
Comprehensive sample preparation is the first line of defense against bioanalytical matrix effects, removing or reducing interferents before mass spectrometry analysis. ResolveMass Laboratories employs multiple extraction techniques tailored to specific matrices and analytes:
Effective Sample Preparation Methods:
- Protein Precipitation: Quick and simple but removes only proteins
- Liquid-Liquid Extraction (LLE): Excellent for removing polar interferents and concentrating analytes
- Solid Phase Extraction (SPE): Selective removal of specific interference classes
- Phospholipid Removal SPE: Specialized sorbents targeting phospholipid elimination
- Supported Liquid Extraction (SLE): Combines LLE selectivity with automation compatibility
ResolveMass Laboratories applies fit-for-purpose extraction strategies as part of our comprehensive bioanalytical services
Chromatographic Separation Enhancement
Improved chromatographic resolution prevents co-elution of matrix components with target analytes. Our laboratory implements:
- Extended gradient programs for critical separations
- Specialized columns (HILIC, mixed-mode, superficially porous particles)
- Two-dimensional LC separation for complex matrices
- Column switching techniques to divert early-eluting interferents
Advanced chromatographic strategies are critical for minimizing bioanalytical matrix effects in both small and large molecule quantification
Matrix-Matched Calibration
Preparing calibration standards in the same biological matrix as study samples ensures that bioanalytical matrix effects affect standards and unknowns equally. This approach, recommended by FDA and EMA guidelines, compensates for matrix interference by maintaining consistent ionization conditions across the analytical run.
Matrix-matched calibration and isotope-labeled internal standards are core components of our IND and NDA bioanalytical services
This approach ensures consistency across clinical and regulatory studies.
Internal Standard Selection and Use
Strategic internal standard selection is critical for compensating residual bioanalytical matrix effects. The ideal internal standard should:
Optimal Internal Standard Characteristics:
- Be a stable isotope-labeled analog of the target analyte (SIL-IS)
- Co-elute exactly with the target analyte
- Experience identical matrix effects
- Be chemically indistinguishable in the ionization source
- Remain stable throughout sample preparation and analysis
ResolveMass Laboratories prioritizes deuterated or 13C-labeled internal standards for regulated bioanalysis, ensuring maximum compensation for matrix variability.
6: Evaluating and Quantifying Matrix Effects
Post-Column Infusion Method
The post-column infusion test is the gold standard for identifying when and where matrix effects occur during chromatographic separation. This technique involves continuously infusing the target analyte into the mass spectrometer while injecting extracted blank matrix. Signal suppression or enhancement appears as negative or positive peaks in the chromatogram, revealing the retention time of interfering matrix components.
We routinely apply post-column infusion during method development to proactively detect matrix effects—an approach embedded across our bioanalytical services outsourcing for pharma
At ResolveMass Laboratories, we perform post-column infusion studies during method development to optimize chromatographic conditions and identify potential co-elution issues before validation.
Matrix Factor Assessment
Regulatory guidelines require quantitative assessment of matrix effects through matrix factor calculations. This involves comparing analyte response in post-extraction spiked samples versus neat solution standards. Matrix factors should fall within 85-115% with acceptable precision (%CV < 15%) across multiple matrix lots to demonstrate adequate control of bioanalytical matrix effects.
Validation Across Multiple Matrix Lots
Testing method performance across 6-10 individual matrix lots from different sources reveals the robustness of the method against biological variability. This approach identifies outlier matrices with exceptional interference and ensures the method performs consistently across the intended patient population.
Robust validation is essential for biotech and pharma sponsors, including those seeking affordable bioanalytical services for biotech startups
7: Regulatory Perspective on Bioanalytical Matrix Effects
Both FDA and EMA bioanalytical method validation guidelines emphasize the importance of evaluating and controlling bioanalytical matrix effects. Regulatory expectations include:
Key Regulatory Requirements:
- Demonstration of matrix effect evaluation during validation
- Use of appropriate internal standards for matrix effect compensation
- Assessment across multiple matrix lots representing study population diversity
- Documentation of matrix interference investigations and resolutions
- Incurred sample reanalysis to confirm real-sample performance
ResolveMass Laboratories maintains full compliance with ICH M10, FDA, and EMA guidelines, ensuring that all validated methods meet the highest regulatory standards for matrix effect control.
Conclusion
Bioanalytical matrix effects are a critical factor in LC-MS/MS analysis that can lead to inaccurate or non-compliant data if not properly managed. These effects – arising from endogenous and exogenous matrix components – can suppress or enhance target signals and thus undermine accuracy, precision, and sensitivity. To achieve reliable results, it is essential to evaluate matrix effects early (using infusion tests, post-spike comparisons, etc.) and mitigate them through optimized sample prep, separation, and calibration strategies.
At ResolveMass Laboratories, our experienced team follows the latest guidelines and best practices to overcome matrix-induced errors. We design assays with built-in controls (like internal standards and matrix-matched curves) and apply targeted cleanup techniques to neutralize interferences. By understanding each matrix’s unique challenges and applying proven solutions, we ensure that bioanalytical matrix effects do not compromise your data’s integrity.
FAQs
A matrix effect occurs when components of the sample other than the target analyte interfere with analytical measurement, altering signal response.
It can impact results by causing inaccurate quantification, poor precision, reduced sensitivity, failed QC samples, and unreliable data, especially in regulated bioanalytical studies.
Key impacts include:
-Ion suppression or enhancement
-Biased analyte concentrations
-Increased variability between samples
-Regulatory non-compliance in bioanalysis
The matrix effect in mass spectrometry refers to changes in analyte ionization efficiency caused by co-eluting matrix components during ionization, particularly in LC-MS/MS.
These components compete with the analyte for charge, leading to ion suppression or ion enhancement.
Common sources in MS include:
-Endogenous lipids
-Salts and phospholipids
-Proteins and metabolites
-Mobile phase additives
There are two primary types of matrix effects, along with one analytical consequence.
1. Ion Suppression
-Decrease in analyte signal
-Most common and most problematic
-Leads to reduced sensitivity and higher LLOQ
2. Ion Enhancement
-Increase in analyte signal
-Can cause overestimation of concentration
-Often less predictable than suppression
3. Matrix-Induced Variability
-Changes in matrix composition between samples
-Leads to poor reproducibility and precision
Matrix effects in HPLC occur when sample components interfere with chromatographic separation or detector response.
Although HPLC itself does not involve ionization, matrix effects can still cause co-elution, peak distortion, baseline noise, and detector saturation.
In HPLC-UV or HPLC-FLD, matrix effects may cause:
-Overlapping peaks
-Reduced resolution
-Inaccurate peak integration
When HPLC is coupled with MS, chromatographic matrix effects directly translate into ionization matrix effects, amplifying analytical risk.
Matrix effects can be reduced by controlling ionization competition and improving sample cleanliness and chromatographic separation.
Best Practices to Minimize Matrix Effects;
-Use stable isotope-labeled internal standards
-Apply matrix-matched calibration curves
-Optimize sample preparation (SPE, LLE, protein precipitation)
-Improve chromatographic separation
-Avoid co-elution with major matrix components
-Evaluate matrix effects during method validation
Regulatory Expectation
FDA, EMA, and ICH M10 guidelines require formal assessment and control of matrix effects in quantitative LC-MS/MS bioanalysis.
Reference
- Matrix effects: the Achilles heel of quantitative high-performance liquid chromatography–electrospray–tandem mass spectrometry.https://www.sciencedirect.com/science/article/abs/pii/S0009912004003364
- Matrix-effect observations in inductively coupled plasma mass spectrometry.https://pubs.rsc.org/en/content/articlelanding/1987/ja/ja9870200745/unauth
- Matrix Effects and Application of Matrix Effect Factor.https://www.tandfonline.com/doi/full/10.4155/bio-2017-0214
- Biological Matrix Effects in Quantitative Tandem Mass Spectrometry-Based Analytical Methods: Advancing Biomonitoring.https://www.tandfonline.com/doi/abs/10.1080/10408347.2014.980775

