Introduction: Why Bioanalysis in Drug Development Is Critical Today
Bioanalysis in drug development plays a foundational role in translating a molecule from discovery to an approved therapeutic. In simple terms, bioanalysis answers one fundamental question: what happens to a drug inside the body over time?
In modern drug development, where molecules are increasingly complex—ranging from small molecules to biologics and long-acting formulations—robust bioanalytical strategies are no longer optional. They are essential for generating reliable, regulatory-acceptable data that guides dosing, safety, and efficacy decisions.
Organizations seeking a specialized bioanalytical CRO in Canada or the U.S. often rely on scientifically driven partners such as ResolveMass Laboratories Inc.
👉 https://resolvemass.ca/bioanalytical-cro-in-canada/
👉 https://resolvemass.ca/bioanalytical-cro-in-the-united-states/
At ResolveMass Laboratories Inc., bioanalysis is approached not just as a testing service, but as a scientific discipline grounded in real-world development experience, regulatory expertise, and advanced analytical science.
👉 https://resolvemass.ca/resolvemass-bioanalytical-services-overview/
Summary
Bioanalysis in drug development serves as the cornerstone of pharmaceutical innovation, enabling precise measurement of drug compounds and their metabolites in biological matrices. This comprehensive guide explores:
- Critical Functions: How bioanalytical methods validate drug safety, efficacy, and pharmacokinetics throughout development phases
- Regulatory Compliance: Meeting FDA, EMA, and ICH guidelines through validated bioanalytical methodologies
- Advanced Technologies: LC-MS/MS, immunoassays, and emerging techniques transforming drug analysis
- Clinical Applications: PK/PD studies, bioequivalence testing, and therapeutic drug monitoring
- Quality Assurance: Method validation, stability testing, and GLP compliance standards
- Future Innovations: AI integration, microsampling techniques, and personalized medicine approaches
Looking for reliable, regulatory-ready bioanalytical services in drug development?
👉 https://resolvemass.ca/bioanalytical-services-in-drug-development/
1: What is Bioanalysis and Why Does It Matter?
Bioanalysis is the measurement and characterization of drugs, metabolites, biomarkers, and biological molecules in complex matrices such as blood, plasma, urine, and tissue. The discipline matters because it generates the pharmacokinetic and pharmacodynamic data that inform critical development decisions.
Companies outsourcing bioanalytical services for pharma and biotech depend on this data to move compounds safely and efficiently through development.
👉 https://resolvemass.ca/bioanalytical-services-outsourcing-for-pharma/
Core Components of Bioanalytical Science
The field encompasses several interconnected elements:
- Sample Preparation: Extraction and purification of target analytes from biological matrices
- Analytical Detection: Utilizing techniques like LC-MS/MS, ELISA, and chromatography for quantification
- Method Validation: Establishing accuracy, precision, and reliability according to regulatory standards
- Data Interpretation: Translating raw analytical results into actionable pharmacological insights
Without scientifically sound bioanalytical quantification, drug developers cannot justify dosing, demonstrate efficacy, or satisfy regulators.
👉 https://resolvemass.ca/bioanalytical-quantification/
2: Regulatory Expectations for Bioanalysis in Drug Development
Bioanalysis in drug development must comply with strict regulatory frameworks to ensure data credibility.
Regulatory-compliant bioanalysis ensures that generated data is accurate, reproducible, and acceptable to global health authorities.
Key Regulatory Guidelines
- US FDA Bioanalytical Method Validation Guidance
- EMA Bioanalytical Method Validation Guideline
- ICH M10 (Bioanalytical Method Validation)
Core Validation Parameters
- Accuracy and precision
- Selectivity and sensitivity
- Matrix effects
- Stability and carryover
ResolveMass Laboratories Inc. designs studies aligned with IND and NDA submission expectations, ensuring inspection-ready documentation and scientifically defensible data.
👉 https://resolvemass.ca/bioanalytical-services-for-ind-nda-submissions/
3: The Critical Role of Bioanalysis in Drug Development Phases
Bioanalysis in drug development supports every phase from discovery through commercialization by providing quantitative evidence of drug behavior in biological systems. Each development stage requires specific bioanalytical applications.
Preclinical Development
During preclinical studies, bioanalytical methods establish:
| Assessment Area | Bioanalytical Application | Key Outcomes |
|---|---|---|
| ADME Studies | Absorption, distribution, metabolism, excretion analysis | Pharmacokinetic profiles |
| Toxicology | Drug and metabolite quantification in animal models | Safety margins and NOAEL determination |
| Formulation Development | Bioavailability testing of different formulations | Optimal delivery systems |
| Metabolite Identification | Structural characterization of biotransformation products | Safety assessments and DDI predictions |
Clinical Development Phases
Phase I Clinical Trials: Bioanalysis in drug development enables first-in-human safety assessments through intensive PK sampling that determines maximum tolerated doses and establishes preliminary safety profiles in healthy volunteers.
Phase II Clinical Trials: Patient population studies utilize bioanalytical data to optimize dosing regimens, confirm therapeutic ranges, and evaluate PK/PD relationships that correlate drug exposure with clinical response.
Phase III Clinical Trials: Large-scale efficacy trials depend on bioanalytical methods for bioequivalence studies, population PK analysis, and confirmation that drug levels achieve therapeutic targets across diverse patient populations.
Post-Market Surveillance
After regulatory approval, bioanalysis continues supporting therapeutic drug monitoring, generic drug bioequivalence testing, and post-marketing safety studies that ensure ongoing patient protection.
After approval, bioanalytical services support therapeutic drug monitoring, generic bioequivalence, and post-marketing safety commitments.
👉 https://resolvemass.ca/bioanalytical-services/
👉 https://resolvemass.ca/bioanalytical-services-2/

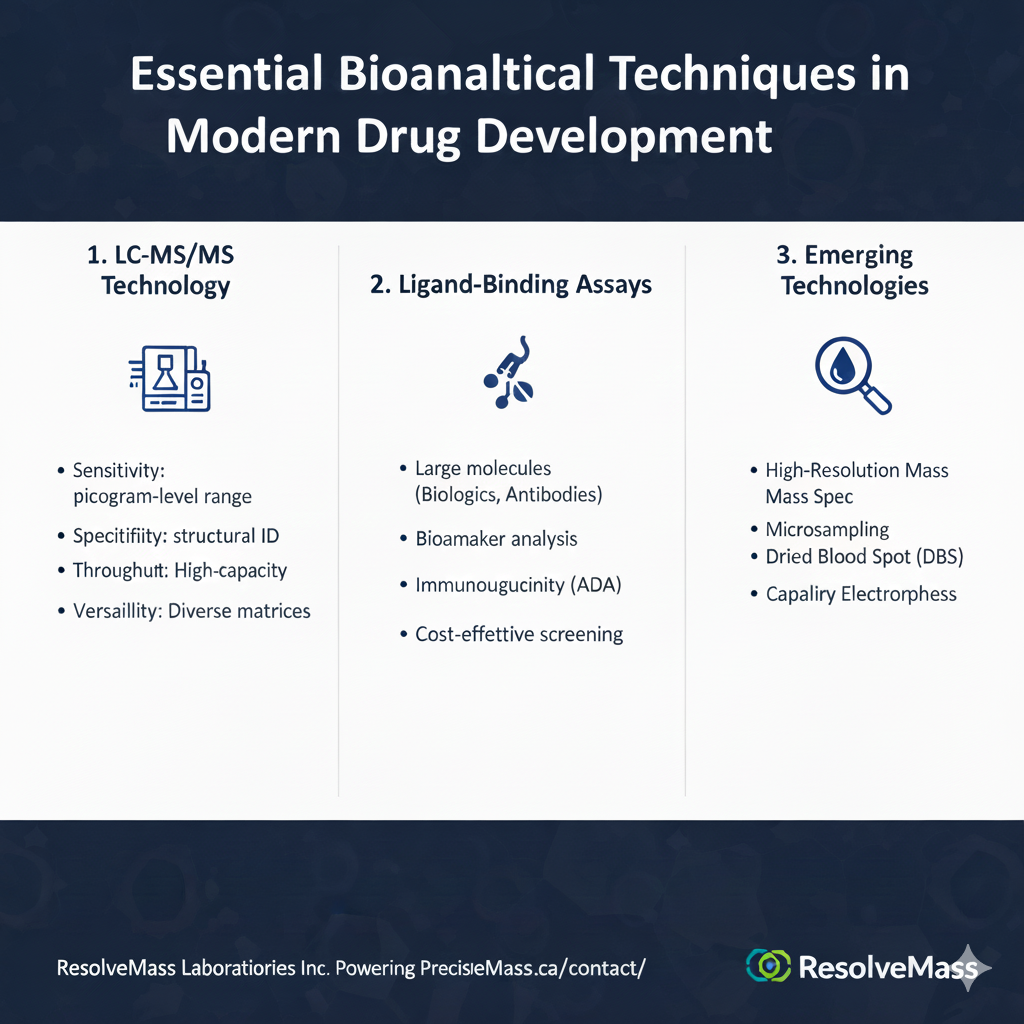
4: Essential Bioanalytical Techniques in Modern Drug Development
The most critical bioanalytical technique in contemporary drug development is liquid chromatography-tandem mass spectrometry (LC-MS/MS), offering unparalleled sensitivity and specificity for small molecule quantification. However, comprehensive programs employ multiple complementary technologies.
1. LC-MS/MS Technology
This gold-standard technique provides:
- Sensitivity: Detection limits in the picogram-per-milliliter range
- Specificity: Structural identification preventing false positives
- Throughput: High-capacity automation supporting large clinical trials
- Versatility: Application to diverse molecular structures and matrices
ResolveMass provides small and large molecule quantification using validated LC-MS/MS workflows.
👉 https://resolvemass.ca/bioanalytical-services-small-large-molecule-quantification/
2. Ligand-Binding Assays
Immunoassays (ELISA, MSD, etc.) excel in:
- Large molecule quantification (biologics, antibodies, proteins)
- Biomarker analysis for patient stratification
- Immunogenicity assessment detecting anti-drug antibodies
- Cost-effective high-throughput screening
👉 https://resolvemass.ca/large-molecule-bioanalysis/
3. Emerging Technologies
Advanced bioanalytical platforms include:
- High-resolution mass spectrometry for metabolite identification
- Microsampling techniques enabling pediatric and volumetric sampling advantages
- Dried blood spot analysis for decentralized clinical trials
- Capillary electrophoresis for charge-variant characterization
Robust bioanalytical method development ensures accuracy across study phases.
👉 https://resolvemass.ca/bioanalytical-method-development-2/
5: The Future of Bioanalysis in Drug Development
Emerging technologies are transforming bioanalysis in drug development through automation, miniaturization, and artificial intelligence integration that accelerate timelines while improving data quality. Several trends are reshaping the field:
1. Automation and High-Throughput Systems
Robotic sample preparation and automated LC-MS/MS systems enable:
- Processing thousands of samples daily
- Reduced human error and improved consistency
- 24/7 operation supporting aggressive timelines
- Resource efficiency for cost-effective development
2. Microsampling Innovations
Reduced sample volume techniques offer:
- Pediatric and neonatal study feasibility
- Frequent sampling without volume constraints
- Remote sample collection for decentralized trials
- Animal welfare improvements in preclinical studies
3. Artificial Intelligence Applications
Machine learning enhances:
- Method development through predictive modeling
- Data analysis identifying patterns and outliers
- Metabolite prediction reducing discovery timelines
- Quality control through real-time monitoring
4. Personalized Medicine Integration
Future bioanalysis will increasingly support:
- Pharmacogenomic-guided dosing
- Real-time therapeutic drug monitoring
- Wearable biosensors for continuous monitoring
- Point-of-care testing enabling immediate adjustments

Conclusion:
Bioanalysis in drug development will continue to evolve as drug modalities become more complex and regulatory scrutiny increases. From early discovery to post-approval monitoring, bioanalysis remains the scientific backbone of informed decision-making.
By combining advanced analytical technology with real-world development expertise, ResolveMass Laboratories Inc. delivers bioanalytical data that sponsors can trust—data that stands up to regulatory review and ultimately protects patient safety.
If you are advancing a molecule through any stage of development, partnering with experienced bioanalytical scientists can significantly improve your program’s success.
FAQs on Bioanalysis in Modern Drug Development
Bioanalysis is a sub-discipline of analytical chemistry providing a quantitative measurement of xenobiotics in various biological matrices. In the world of drug development, ensuring a drug’s safety, efficacy, and optimal performance is paramount. Bioanalysis plays a pivotal role in achieving these goals.
Key Drug Discovery Applications
Biostatistics guides experiment design and sample-size decisions for animal and cell-based models. By evaluating dose response, toxicity, and survival, researchers assess a drug’s potential efficacy prior to clinical trials.
Bioanalysis in drug development enables accurate measurement of drug concentrations over time, forming the basis of PK/PD modeling.
Through sensitive and selective assays, bioanalysis allows calculation of key parameters such as AUC, Cmax, half-life, clearance, and exposure–response relationships. These data guide dose optimization and help correlate systemic exposure with therapeutic or toxic effects.
Bioanalytical method validation ensures that analytical methods are accurate, precise, and reliable for their intended use.
Validation typically evaluates parameters such as selectivity, sensitivity, accuracy, precision, recovery, matrix effects, stability, and carryover, in alignment with FDA, EMA, and ICH M10 guidelines. Proper validation is critical for regulatory acceptance of study data.
Regulatory compliance ensures that bioanalysis data is credible, reproducible, and acceptable to health authorities.
Non-compliant bioanalytical data can lead to study rejection, delays, or additional trials. Adherence to global guidelines and inspection-ready documentation is essential for successful submissions.
Reference
- Bioanalysis in drug discovery and development.Pharmaceutical Methods.https://www.sciencedirect.com/science/article/abs/pii/S2229470810110036
- Changing need for bioanalysis during drug development.https://analyticalsciencejournals.onlinelibrary.wiley.com/doi/abs/10.1002/bmc.932
- Roles of LC-MS Bioanalysis in Drug Discovery, Development, and Therapeutic Drug Monitoring.https://onlinelibrary.wiley.com/doi/abs/10.1002/9781118671276.ch1
- Bioanalytical method development and validation: Critical concepts and strategies.https://www.sciencedirect.com/science/article/abs/pii/S1570023216308881
- Chapter 1 – Bioanalysis: methods, techniques, and applications.Analytical Techniques in Biosciences.https://www.sciencedirect.com/science/chapter/edited-volume/abs/pii/B9780128226544000026

