Introduction
The pharmaceutical industry faces unprecedented challenges in bringing new therapeutics to market, with average development timelines exceeding 10-15 years and costs surpassing $2.6 billion per approved drug. In this demanding landscape, partnering with a specialized bioanalytical CRO for drug discovery has become essential for pharmaceutical and biotechnology companies seeking to optimize their early-stage research programs. A bioanalytical Contract Research Organization (CRO) provides critical analytical support that transforms raw biological samples into actionable data, enabling informed decision-making during the most crucial phases of drug development.
A bioanalytical Contract Research Organization (CRO) provides critical analytical support that converts biological samples into actionable data through comprehensive bioanalytical services. This data enables informed decision-making during the most crucial phases of drug discovery and preclinical development.
ResolveMass Laboratories Inc. supports discovery and preclinical programs through advanced platforms including LC-MS/MS bioanalysis of xenobiotics, regulated workflows, and fit-for-purpose assay strategies that bridge discovery and regulated bioanalysis.
Summary
Key Takeaways:
- A bioanalytical CRO for drug discovery accelerates early pharmaceutical development through specialized analytical expertise.
- Outsourcing bioanalytical services in drug development reduces cost, improves data quality, and shortens timelines.
- Core services include bioanalytical method development, bioanalytical method validation, PK/PD bioanalysis, and biomarker quantification.
- Early CRO engagement minimizes late-stage failures and regulatory delays.
- ResolveMass Laboratories Inc. provides scalable, compliant solutions for discovery-stage and preclinical programs.
1: Understanding Bioanalytical CROs in the Drug Discovery Ecosystem
Bioanalytical CROs serve as specialized partners that bridge the gap between drug discovery research and clinical development. These organizations provide comprehensive analytical services that support lead identification, optimization, and preclinical safety assessment.
A bioanalytical CRO for drug discovery serves as a specialized partner that bridges discovery research and regulated development. These organizations deliver high-quality analytical data that supports lead identification, optimization, and preclinical safety assessment through dedicated bioanalytical laboratory services.
What Makes a Bioanalytical CRO Essential?
A bioanalytical CRO for drug discovery delivers specialized expertise and infrastructure that most pharmaceutical companies cannot efficiently maintain in-house. The primary value proposition includes:
- Technical Expertise: Access to scientists with decades of combined experience in complex bioanalytical quantification
- Advanced Instrumentation: State-of-the-art LC-MS/MS, HRMS, and ligand-binding assay platforms
- Regulatory Knowledge: Deep understanding of FDA, EMA, and ICH guidelines for regulated bioanalytical services
- Scalability: Flexible capacity enabled by bioanalytical outsourcing
- Cost Efficiency: Cost-efficient models, especially for biotech startups
Core Services Provided During Early Development
| Service Category | Application in Drug Discovery | Timeline Impact |
|---|---|---|
| Method Development | Creating sensitive, selective assays for drug quantification | 2-4 weeks |
| Method Validation | Ensuring analytical procedures meet regulatory standards | 4-8 weeks |
| Sample Analysis | Quantifying drugs and metabolites in biological matrices | Variable |
| Metabolite Identification | Characterizing biotransformation pathways | 2-6 weeks |
| Biomarker Analysis | Measuring pharmacodynamic and safety markers | 3-8 weeks |
| PK/TK Studies | Determining absorption, distribution, metabolism, and excretion | 6-12 weeks |
2: The Critical Stages Where Bioanalytical CROs Impact Drug Discovery
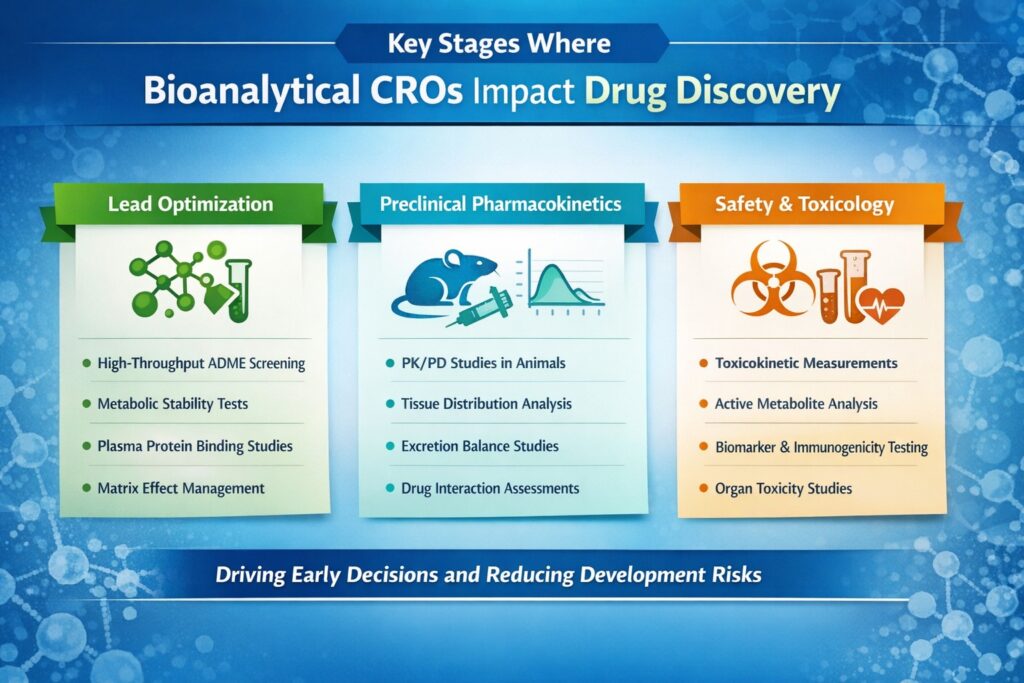
1. Lead Identification and Optimization Phase
During lead identification and optimization, a bioanalytical CRO for drug discovery delivers rapid analytical data using high-throughput bioanalysis. These studies support early ADME profiling, exposure assessments, and metabolite characterization.
Key activities include:
- High-throughput screening support for in vitro ADME assays
- Plasma protein binding studies to predict free drug concentrations
- Metabolic stability assessments using liver microsomes and hepatocytes
- Navigating discovery vs regulated bioanalysis
- Permeability studies to evaluate blood-brain barrier penetration and oral bioavailability
- Early exposure assessments in discovery toxicology studies
- Managing bioanalytical matrix effects
- Addressing common challenges in bioanalytical method development
By providing rapid turnaround analytical data, ResolveMass Laboratories Inc. enables medicinal chemistry teams to make real-time decisions about structure-activity relationships and optimize pharmacokinetic properties before advancing candidates to formal preclinical development.
2. Preclinical Pharmacokinetic Characterization
Reliable PK/PD data generated through PK/PD bioanalysis is essential for dose selection and translational modeling. A bioanalytical CRO for drug discovery ensures analytical consistency across matrices, species, and study designs.
Essential PK services include:
- Single and multiple dose PK studies in rodents and non-rodents
- Tissue distribution studies to understand drug accumulation patterns
- Excretion balance studies using radiolabeled compounds
- Drug-drug interaction potential assessments
- Food effect evaluations in relevant animal models
The quality of bioanalytical data generated during preclinical PK studies directly influences the design of first-in-human clinical trials and the accuracy of human dose predictions.
3. Safety Pharmacology and Toxicology Support
A bioanalytical CRO for drug discovery plays an indispensable role in preclinical safety assessment by quantifying drug and metabolite exposure in toxicology studies. This data establishes critical exposure-response relationships that inform safety margins and clinical dosing strategies.
Through specialized toxicokinetic bioanalysis, CROs quantify exposure in GLP toxicology studies to establish safety margins and support IND-enabling packages.
Toxicokinetic support includes:
- Parent drug quantification in GLP-compliant toxicology studies
- Active metabolite measurements to ensure adequate coverage in animal species
- Biomarker analysis for early detection of organ toxicity
- Immunogenicity assessments for biological therapeutics
- Analysis of samples from specialized safety studies (cardiovascular, respiratory, CNS)
According to industry data, approximately 30% of drug development failures occur due to inadequate safety profiles identified during preclinical or clinical stages. Early identification of safety concerns through comprehensive bioanalytical characterization significantly reduces the risk of costly late-stage attrition.
3: Advanced Analytical Technologies Employed by Bioanalytical CROs
1. Mass Spectrometry-Based Approaches
Modern CROs rely on advanced LC-MS platforms to support small molecule vs large molecule bioanalysis and emerging therapeutic modalities. ResolveMass also supports LC-MS for large molecules and complex biologics.
Key technologies include:
- LC-MS/MS (Triple Quadrupole): Gold standard for quantitative bioanalysis with detection limits in the pg/mL range
- High-Resolution Mass Spectrometry (HRMS): Enables accurate mass measurement for metabolite identification and confirmation
- Trapped Ion Mobility Spectrometry (TIMS): Provides additional separation dimension for complex biological matrices
- Microflow and Nanoflow LC-MS: Ultra-sensitive analysis for limited sample volumes
ResolveMass Laboratories Inc. maintains state-of-the-art instrumentation calibrated and qualified to meet stringent regulatory requirements, ensuring data quality that withstands regulatory scrutiny.
2. Complementary Analytical Platforms
While mass spectrometry dominates small molecule bioanalysis, complementary technologies provide unique advantages for specific applications:
- Ligand-Binding Assays (ELISA, MSD): Optimal for large molecule therapeutics and protein biomarkers
- Flow Cytometry: Critical for cellular biomarkers and immunophenotyping
- qPCR and RNA-seq: Essential for pharmacodynamic gene expression studies
- Immunohistochemistry: Provides spatial context for tissue drug distribution
- Biosimilar bioanalysis
4: Ensuring Regulatory Compliance and Data Integrity
A trusted bioanalytical CRO for drug discovery operates in alignment with regulatory expectations for IND and NDA submissions. ResolveMass supports sponsors through bioanalytical services for IND/NDA submissions and clinical bioanalytical services.
1. Adherence to Bioanalytical Guidelines
A reputable bioanalytical CRO operates in strict accordance with regulatory guidelines that govern method validation and sample analysis. The primary guidance documents include:
- FDA Guidance for Industry: Bioanalytical Method Validation (2018)
- EMA Guideline on Bioanalytical Method Validation (2011)
- ICH M10 Guideline on Bioanalytical Method Validation (2022)
These guidelines establish acceptance criteria for accuracy, precision, selectivity, sensitivity, reproducibility, and stability that must be demonstrated during method validation.
2. Quality Management Systems
ResolveMass Laboratories Inc. maintains comprehensive quality management systems that ensure data integrity throughout the analytical process:
- 21 CFR Part 11 Compliance: Electronic records and signatures systems with full audit trails
- GLP Compliance: Quality assurance oversight for regulatory toxicology studies
- ISO Certification: International quality standards for laboratory operations
- Data Backup and Security: Redundant systems protecting against data loss
- Chain of Custody Procedures: Sample tracking from receipt through disposal
These capabilities extend across bioanalytical services in North America with robust data integrity systems and audit-ready documentation.
5: Strategic Advantages of Early CRO Engagement
Early engagement with a bioanalytical CRO for drug discovery via outsourced bioanalytical services enables parallel development, faster timelines, and reduced program risk.
Sponsors also gain clarity around bioanalytical testing services cost and benefit from scalable models offered through bioanalytical services outsourcing for pharma.
1. Accelerating Development Timelines
Pharmaceutical companies that engage a bioanalytical CRO for drug discovery during early development phases experience significant timeline advantages. Early analytical support enables:
- Parallel rather than sequential execution of critical studies
- Immediate identification of problematic drug candidates
- Proactive method development before formal preclinical studies
- Reduced rework from analytical method failures
Industry benchmarking studies demonstrate that early CRO engagement can compress preclinical development timelines by 6-12 months compared to sequential internal development approaches.
2. Cost Optimization Strategies
The financial benefits of partnering with a specialized bioanalytical CRO extend beyond simple outsourcing economics:
Direct cost savings:
- Elimination of capital equipment purchases ($500K-$2M per instrument)
- Reduced personnel costs through variable staffing models
- Lower overhead allocation compared to internal operations
- Decreased consumables waste through optimized method efficiency
Indirect cost savings:
- Earlier identification of unsuitable candidates prevents wasteful downstream investment
- Higher quality data reduces regulatory review cycles and approval delays
- Faster time-to-market generates additional revenue years
Financial modeling indicates that strategic CRO partnerships typically reduce preclinical development costs by 30-40% while simultaneously improving data quality and regulatory compliance.
6: Selecting the Right Bioanalytical CRO Partner
When selecting a bioanalytical CRO for drug discovery, sponsors should evaluate:
- Breadth of capabilities outlined in the ResolveMass bioanalytical services overview
- Proven experience as a dedicated bioanalytical CRO
- Scientific depth across PK, TK, biomarkers, and regulated studies
- Clear understanding of why bioanalysis is important
1. Essential Evaluation Criteria
Choosing a bioanalytical CRO for drug discovery requires careful assessment of multiple factors that impact project success:
Technical Capabilities:
- Breadth of analytical platforms and technologies
- Experience with relevant therapeutic areas and molecular modalities
- Demonstrated method development success for challenging compounds
- Capacity for specialized studies (tissue analysis, imaging mass spectrometry, etc.)
Regulatory Expertise:
- Track record of successful regulatory submissions
- GLP certification and inspection history
- Understanding of global regulatory requirements
- Quality system maturity and CAPA effectiveness
Operational Excellence:
- Realistic timeline commitments and on-time delivery performance
- Transparent communication and project management practices
- Flexibility to accommodate protocol amendments and scope changes
- Competitive pricing without compromising quality standards
2. Red Flags to Avoid
Organizations evaluating bioanalytical CRO partners should be cautious of:
- Promises of unrealistic timelines that compromise data quality
- Lack of regulatory inspection history or unsuccessful audit outcomes
- Inadequate data integrity systems or poorly documented procedures
- Limited technical depth or overreliance on inexperienced personnel
- Poor communication responsiveness during the evaluation phase
Conclusion
The role of a bioanalytical CRO for drug discovery extends far beyond simple analytical testing to encompass strategic partnership that fundamentally improves the efficiency, quality, and success rate of pharmaceutical development programs. During early drug discovery and preclinical development, when critical decisions about candidate advancement are made, the analytical data provided by specialized CROs serves as the foundation for informed decision-making.
By leveraging advanced analytical technologies, regulatory expertise, and operational excellence, bioanalytical CROs enable pharmaceutical and biotechnology companies to accelerate timelines, optimize costs, and increase the probability of bringing safe and effective therapeutics to patients. The strategic value of early CRO engagement cannot be overstated—organizations that integrate bioanalytical CRO expertise from the earliest stages of discovery consistently outperform those that delay such partnerships.
ResolveMass Laboratories Inc. stands ready to support your drug discovery and preclinical development programs with the analytical expertise, technological capabilities, and quality systems necessary for success. As the pharmaceutical industry continues to evolve toward more complex molecular modalities and precision medicine approaches, the importance of specialized bioanalytical CRO for drug discovery partnerships will only increase.
Frequently Asked Questions:
The role of a CRO in drug development is to provide specialized scientific, technical, and regulatory services that support pharmaceutical and biotech companies from early discovery through clinical trials.
CROs assist with bioanalysis, preclinical studies, clinical trial management, data analysis, and regulatory documentation, helping sponsors reduce timelines, control costs, and ensure compliance.
The function of a CRO sponsor in clinical trials is to act on behalf of the trial sponsor by managing operational and scientific aspects of the study while the sponsor retains overall responsibility.
CROs may oversee site management, monitoring, data collection, bioanalytical testing, and regulatory compliance under a formal agreement with the sponsor.
CROs play a critical role in clinical trials by planning, executing, and monitoring studies in accordance with regulatory and ethical standards.
Their responsibilities include patient recruitment support, clinical monitoring, bioanalytical testing, data management, statistical analysis, and ensuring trial quality and integrity.
A bioanalytical CRO for drug discovery is a specialized contract research organization that provides analytical testing services to support early drug discovery and preclinical development.
These CROs generate high-quality data through method development, PK/PD bioanalysis, ADME studies, biomarker analysis, and toxicokinetic testing to guide informed decision-making before clinical trials.
A bioanalytical CRO supports lead identification and optimization by delivering rapid, accurate analytical data that helps prioritize the most promising drug candidates.
Key contributions include in vitro ADME testing, metabolic stability studies, plasma protein binding analysis, permeability assessments, and early exposure measurements that enable structure-activity relationship optimization.
Bioanalytical data directly impacts first-in-human dose selection by defining drug exposure, safety margins, and exposure-response relationships in preclinical models.
Accurate PK and toxicokinetic data generated by a bioanalytical CRO enable confident starting dose calculations, reduce safety risks, and improve the likelihood of successful early clinical trials.
Reference
- Saurabh Pand.ey , Preeti Pandey , Gaurav Tiwari , Ruchi Tiwari.Bioanalysis in drug discovery and development.https://www.sciencedirect.com/science/article/abs/pii/S2229470810110036
- Aarzoo Thakur,Zhiyuan Tan,Tsubasa Kameyama,Eman El-Khateeb,Shakti Nagpal,Stephanie Malone,Rohitash Jamwal &Chukwunonso K. Nwabufo.Bioanalytical strategies in drug discovery and development.https://www.tandfonline.com/doi/abs/10.1080/03602532.2021.1959606
- Nuggehally R. Srinivas.Changing need for bioanalysis during drug development.https://analyticalsciencejournals.onlinelibrary.wiley.com/doi/abs/10.1002/bmc.932
- Olivia Seifert.The role of digital data management in today’s collaborative drug discovery approach.https://www.tandfonline.com/doi/full/10.4155/bio-2023-0008
- Roles of LC-MS Bioanalysis in Drug Discovery, Development, and Therapeutic Drug Monitoring.https://onlinelibrary.wiley.com/doi/abs/10.1002/9781118671276.ch1
- Applicability of bioanalysis of multiple analytes in drug discovery and development: review of select case studies including assay development considerations.https://analyticalsciencejournals.onlinelibrary.wiley.com/doi/abs/10.1002/bmc.594

