Introduction
Bioanalytical method development represents the cornerstone of pharmaceutical research and drug development in the United States. This critical process involves creating and validating analytical techniques that accurately measure drug compounds and their metabolites in biological samples such as blood, plasma, urine, and tissue. For pharmaceutical companies, biotechnology firms, and contract research organizations (CROs), robust bioanalytical method development ensures regulatory compliance, accelerates drug approval timelines, and ultimately brings life-saving therapies to patients faster.
At ResolveMass Laboratories Inc., we understand that the success of any pharmaceutical development program hinges on the quality and reliability of bioanalytical data. With over a decade of experience supporting drug development programs across North America, our team has developed and validated hundreds of methods for small molecules, biologics, and biosimilars, helping clients navigate the complex regulatory landscape while maintaining the highest scientific standards.
Summary
- Bioanalytical method development is the systematic process of creating validated analytical procedures to measure drug concentrations in biological matrices
- U.S. laboratories follow strict FDA guidelines ensuring accuracy, precision, and regulatory compliance
- Key techniques include LC-MS/MS, ELISA, immunoassays, and advanced chromatography methods
- Method validation involves specificity, sensitivity, linearity, accuracy, precision, and stability testing
- ResolveMass Laboratories Inc. provides comprehensive bioanalytical services supporting pharmaceutical development from early discovery through clinical trials
- Proper method development reduces costly delays and ensures reliable data for regulatory submissions
1: What is Bioanalytical Method Development?
Bioanalytical method development is the systematic creation of analytical procedures designed to quantify drugs, metabolites, biomarkers, or biological molecules in complex biological matrices. The process begins immediately after a pharmaceutical compound shows promise in early research and continues throughout clinical development.
Bioanalytical method development is the process of designing, validating, and applying analytical techniques to quantify drugs, metabolites, and biomarkers in biological matrices such as plasma, serum, and tissue. This process ensures accurate, reliable, and reproducible results critical for drug discovery, clinical trials, and regulatory submissions.
The primary objectives include:
- Establishing reliable quantification methods for drug concentrations
- Ensuring methods meet FDA and regulatory requirements
- Determining pharmacokinetic and pharmacodynamic properties
- Supporting dose selection and safety assessments
- Enabling toxicokinetic studies in preclinical development
Importance in the Pharmaceutical Industry
Bioanalytical method development ensures compliance with FDA and EMA guidelines while supporting drug efficacy and safety evaluations. Its applications span drug metabolism, pharmacokinetics, and toxicokinetics studies.
2: Key Techniques in Bioanalytical Method Development
Several advanced techniques are employed to achieve precision and reliability in bioanalytical testing:
1. Liquid Chromatography-Mass Spectrometry (LC-MS/MS)
LC-MS/MS has become the gold standard for bioanalytical method development in the pharmaceutical industry. This technique offers unparalleled sensitivity, specificity, and speed, making it ideal for quantifying small molecule drugs and their metabolites.
Advantages of LC-MS/MS:
| Feature | Benefit |
|---|---|
| High Sensitivity | Detection limits in picogram/milliliter range |
| Selectivity | Distinguishes compounds from biological interference |
| Speed | Rapid analysis supporting high-throughput studies |
| Versatility | Applicable to diverse compound types |
| Regulatory Acceptance | Widely accepted by FDA and global authorities |
At ResolveMass Laboratories Inc., our state-of-the-art LC-MS/MS platforms enable us to develop methods with lower limits of quantification (LLOQ) as low as 0.1 ng/mL, ensuring we can detect therapeutic concentrations even for highly potent compounds.
2. Enzyme-Linked Immunosorbent Assay (ELISA)
ELISA remains essential for large molecule analysis, particularly for biotherapeutics such as monoclonal antibodies, fusion proteins, and peptides. This immunoassay technique leverages the specificity of antibody-antigen interactions to quantify biological drugs in complex matrices.
When ELISA is preferred:
- Analysis of protein therapeutics and biologics
- Immunogenicity assessments (anti-drug antibody detection)
- Biomarker quantification
- High-throughput screening applications
- Cost-effective method for large sample sets
3. Ligand Binding Assays (LBA)
Beyond traditional ELISA, advanced ligand binding assays including electrochemiluminescence (ECL) and homogeneous assays provide enhanced sensitivity and broader dynamic ranges for bioanalytical method development projects involving biologics and biosimilars.
4. High-Performance Liquid Chromatography (HPLC)
While LC-MS/MS dominates modern bioanalysis, HPLC with UV, fluorescence, or electrochemical detection remains valuable for certain applications, particularly when mass spectrometry is unavailable or unnecessary for highly abundant analytes.

3: The Bioanalytical Method Development Process
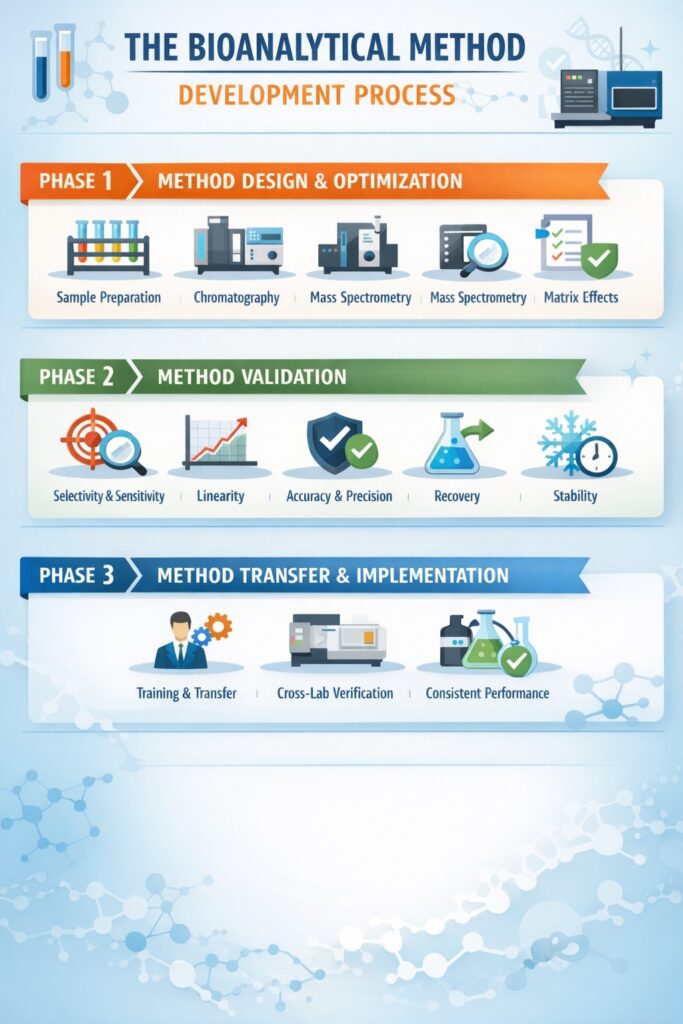
Phase 1: Method Design and Optimization
The journey begins with understanding the analyte’s chemical properties, therapeutic concentration range, and matrix effects. Our scientists evaluate multiple extraction techniques, chromatographic conditions, and detection parameters to identify the optimal approach.
Critical considerations include:
- Sample preparation strategy (protein precipitation, liquid-liquid extraction, solid-phase extraction)
- Chromatographic separation conditions
- Mass spectrometer parameters and ionization mode
- Internal standard selection
- Matrix effects mitigation
Phase 2: Method Validation
Method validation for bioanalytical method development follows stringent FDA guidance documents, particularly the 2018 Bioanalytical Method Validation Guidance for Industry. This comprehensive validation ensures the method produces reliable, reproducible data suitable for regulatory submissions.
FDA-required validation parameters:
- Selectivity/Specificity: Ensures the method measures only the intended analyte without interference
- Sensitivity: Establishes the lower limit of quantification (LLOQ) with acceptable accuracy and precision
- Linearity: Demonstrates proportional response across the calibration range (typically r ≥ 0.99)
- Accuracy: Within ±15% of nominal concentrations (±20% at LLOQ)
- Precision: Intra-day and inter-day variability ≤15% CV (≤20% at LLOQ)
- Recovery: Consistent extraction efficiency across concentration ranges
- Stability: Freeze-thaw, bench-top, long-term, and stock solution stability
Phase 3: Method Transfer and Implementation
Once validated, methods must be successfully transferred to bioanalytical laboratories conducting sample analysis. ResolveMass Laboratories Inc. employs comprehensive technology transfer protocols ensuring seamless method implementation and consistent performance across studies.
4: Applications of Bioanalytical Method Development
Bioanalytical method development extends across numerous industries and applications:
1. Preclinical Development
During preclinical studies, bioanalytical method development supports toxicokinetic assessments in animal models, helping determine safe starting doses for human trials and identifying potential safety concerns before clinical development begins.
2. Clinical Pharmacology
In Phase I through Phase III clinical trials, validated bioanalytical methods enable:
- Pharmacokinetic (PK) profiling to understand drug absorption, distribution, metabolism, and elimination
- Pharmacodynamic (PD) assessments linking drug exposure to therapeutic effects
- Dose-response relationship characterization
- Drug-drug interaction studies
- Special population assessments (pediatric, geriatric, hepatic/renal impairment)
3. Bioequivalence Studies
For generic drug development, highly sensitive and specific bioanalytical methods are essential for demonstrating bioequivalence to reference products, supporting abbreviated new drug applications (ANDAs).
4. Biosimilar Development
The complexity of biological drugs demands sophisticated analytical strategies. Our bioanalytical method development expertise extends to biosimilars, where we develop comprehensive analytical packages comparing candidate products to reference biologics.
5: Challenges in Bioanalytical Method Development
Despite advancements, bioanalytical method development faces several challenges:
1. Matrix Effects
Biological matrices contain thousands of endogenous compounds that can interfere with analyte detection. Overcoming matrix effects requires careful method optimization, appropriate sample preparation, and thorough validation to ensure accuracy across diverse sample types.
2. Metabolite Analysis
Many drugs undergo extensive metabolism, creating multiple metabolites that may contribute to efficacy or toxicity. Developing methods that simultaneously quantify parent drugs and key metabolites presents significant technical challenges requiring advanced chromatographic separation and selective detection.
3. Low Concentration Quantification
Modern therapeutics increasingly include highly potent compounds requiring detection at extremely low concentrations. Achieving sub-nanogram per milliliter sensitivity while maintaining accuracy and precision demands cutting-edge instrumentation and expert method development.
4. Stability Challenges
Certain compounds are inherently unstable in biological matrices, degrading during collection, storage, or analysis. Addressing stability issues may require specialized collection procedures, stabilizing agents, or rapid processing protocols.
6: Services Offered by Bioanalytical CROs in the United States
Contract Research Organizations (CROs) play a pivotal role in bioanalytical method development. Key services include:
1. Method Development and Validation
CROs offer tailored method development and validation services, ensuring methods meet regulatory guidelines.
2. Sample Analysis
High-throughput analysis of biological samples using advanced instrumentation.
3. Pharmacokinetics and Toxicokinetics Studies
CROs provide comprehensive PK/PD studies to support drug development programs.
4. Biomarker Analysis
From discovery to validation, CROs support biomarker-driven studies essential for precision medicine.
5. Regulatory Support
Expert consultation and support for regulatory submissions ensure compliance and expedite approval processes.
7: Why Choose the United States for Bioanalytical Method Development?
The United States offers several advantages for bioanalytical method development:
1. Regulatory Expertise
The FDA’s stringent standards foster innovation and ensure high-quality bioanalytical methods.
2. Access to Advanced Technologies
U.S.-based labs have access to cutting-edge technologies and highly skilled professionals.
3. Thriving Pharmaceutical and Biotech Ecosystem
Home to global pharmaceutical leaders, the U.S. provides an ecosystem conducive to bioanalytical advancements.
Conclusion
Bioanalytical method development serves as the foundation for successful pharmaceutical development programs in the United States. From early discovery through regulatory approval and post-market surveillance, robust analytical methods ensure the reliable data necessary for decision-making, regulatory compliance, and ultimately, patient safety.
Bioanalytical method development is a cornerstone of modern drug discovery, precision medicine, and regulatory compliance. The United States continues to lead in innovation, offering unparalleled expertise and technologies. With services ranging from method development to biomarker analysis, bioanalytical testing remains indispensable for pharmaceutical, biotech, and related industries.
ResolveMass Laboratories Inc. stands ready to support your bioanalytical needs with comprehensive services, cutting-edge technology, and deep regulatory expertise. Our commitment to scientific excellence, quality, and client collaboration has made us a trusted partner for pharmaceutical and biotechnology companies across North America.
References
- Food and Drug Administration (FDA). Bioanalytical Method Validation Guidance.
- ICH. Guidelines on Bioanalytical Method Validation
- Mohammad Mahdi Moein ,Aziza El Beqqali, Mohamed Abdel-Rehim.Bioanalytical method development and validation: Critical concepts and strategies.https://www.sciencedirect.com/science/article/abs/pii/S1570023216308881
- The history of bioanalytical method validation and regulation: Evolution of a guidance document on bioanalytical methods validation.https://pmc.ncbi.nlm.nih.gov/articles/PMC2751303/
- Mithun Rudrapal , Aniket P. Kothawade , Shahira M. Ezzat , Chukwuebuka Egbuna.Chapter 1 – Bioanalysis: methods, techniques, and applications.https://www.sciencedirect.com/science/chapter/edited-volume/abs/pii/B9780128226544000026
- International Guidelines for Bioanalytical Method Validation: A Comparison and Discussion on Current Scenario.https://link.springer.com/article/10.1007/s10337-010-1869-2
How to Build a Virtual Chemistry Department Using an Outsourced CRO Model
Summary Building a Virtual Chemistry Department through an outsourced CRO model allows biotech and pharma…
Drug Discovery CRO Chemistry Services: What Early-Stage Biotechs Should Expect
🔍 Summary at a Glance Clear deliverables, timelines, and communication protocols are critical when outsourcing…
End-to-End Biomarker Bioanalytical Services Offered by a CRO: Capabilities and Expectations
Introduction Biomarker bioanalytical services CRO partnerships have become indispensable in modern pharmaceutical development, providing the…
Regulatory-Compliant Biomarker Bioanalytical Services for FDA and Health Canada Submissions
Introduction Biomarker bioanalytical services for FDA and Health Canada submissions require rigorous compliance with regulatory…
Outsourced Medicinal Chemistry for Startups: Cost, Speed, and Scientific Advantages
🔍 Summary (Key Takeaways) Outsourced medicinal chemistry for startups offers a strategic advantage by minimizing…
Why Early-Stage Biotech Companies Outsource Chemistry Services to Drug Discovery CROs
Introduction Early-stage biotech companies are increasingly adopting Outsourced Chemistry Services for Biotech to stay competitive…