Introduction:
Bioanalytical Method Transfer is the structured process of transferring a validated bioanalytical method from one laboratory to another while ensuring equivalent performance and regulatory compliance.
For emerging biotechs advancing drug candidates into IND-enabling or clinical studies, Bioanalytical Method Transfer is not just a technical exercise—it is a regulatory milestone. Improper transfer can compromise pharmacokinetic (PK), toxicokinetic (TK), or biomarker datasets, potentially delaying interactions with regulators such as the U.S. Food and Drug Administration or European Medicines Agency.
At ResolveMass Laboratories Inc., we have supported multiple biotech programs through:
- Bioanalytical Method Development
- Rapid Bioanalytical Method Development
- Bioanalytical Method Validation
- IND Enabling Bioanalytical Studies
- Bioanalytical Services for IND & NDA Submissions
—ensuring data continuity across laboratories and phases of development.
Summary:
- Bioanalytical Method Transfer ensures a validated method performs equivalently at a new laboratory.
- Cross-validation confirms data comparability between original and receiving labs.
- Regulatory agencies like the U.S. Food and Drug Administration and Health Canada expect documented evidence of accuracy, precision, and reproducibility.
- Poorly executed transfers can delay IND, CTA, or BLA submissions.
- Early planning, predefined acceptance criteria, and statistical evaluation are critical.
- Experienced CRO partners reduce compliance risk and protect program timelines.
For a broader understanding of why bioanalysis is central to drug development.
1: What is Bioanalytical Method Transfer?
Bioanalytical Method Transfer is the documented demonstration that a receiving laboratory can reproduce the performance characteristics of a validated method originally developed elsewhere.
It typically occurs when:
- A biotech transitions from discovery to GLP studies
- Clinical phases require higher sample throughput
- A sponsor changes CROs
- A method moves from in-house to external laboratory
- A merger or acquisition changes analytical sites
Related service overview:
https://resolvemass.ca/resolvemass-bioanalytical-services-overview/
The goal is clear: Maintain data integrity and regulatory defensibility.
2: Why is Bioanalytical Method Transfer Critical for Regulatory Success ?
Regulatory agencies require evidence that transferred methods maintain accuracy, precision, selectivity, sensitivity, and stability performance.
Under guidance from the U.S. Food and Drug Administration and International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use, sponsors must demonstrate:
- Reproducibility between laboratories
- Comparable calibration curve performance
- Equivalent QC acceptance rates
- Consistent LLOQ performance
- Stability confirmation
Failure to properly execute Bioanalytical Method Transfer may result in:
- Regulatory queries
- Data rejection
- Repeat studies
- Delayed IND/CTA approvals
For common compliance pitfalls, see:
https://resolvemass.ca/common-bioanalytical-mistakes/
https://resolvemass.ca/data-integrity-in-bioanalytical-studies/
3: Bioanalytical Method Transfer VS Cross-Validation
Bioanalytical Method Transfer ensures method reproducibility in a new lab, while cross-validation compares results between two methods or labs to confirm data comparability.
| Parameter | Bioanalytical Method Transfer | Cross-Validation |
|---|---|---|
| Purpose | Demonstrate method reproducibility | Compare data sets between labs or methods |
| When Required | Lab change or site expansion | Multiple labs or methodological changes |
| Samples Used | Spiked QC samples | Study samples and QCs |
| Regulatory Focus | Method performance | Data comparability |
Both processes may be required in multi-site global trials or outsourced models:
https://resolvemass.ca/outsourced-bioanalysis-for-drug-development/
https://resolvemass.ca/managing-bioanalytical-cro-projects/
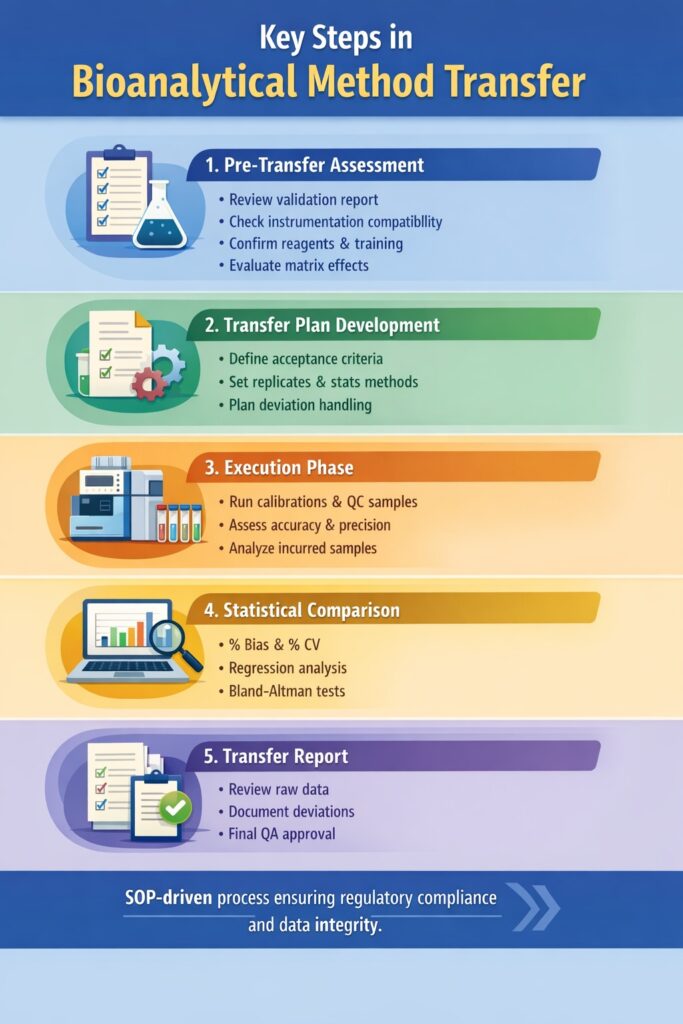
4: Key Steps in Bioanalytical Method Transfer
A successful Bioanalytical Method Transfer follows a structured, documented workflow.
1. Pre-Transfer Assessment
The pre-transfer phase evaluates whether the receiving laboratory can technically reproduce the validated method without introducing bias.
This stage includes:
- Review of the original method validation report
- Evaluation of instrumentation compatibility (e.g., LC-MS/MS platforms)
- Confirmation of reagent and reference standard equivalence
- Assessment of analyst training and competency
- Review of SOP alignment between laboratories
Matrix & Extraction Considerations
Matrix effects and extraction recovery are common sources of variability during Bioanalytical Method Transfer. Proper evaluation of ion suppression/enhancement and recovery consistency is critical.
A comprehensive feasibility assessment reduces the likelihood of transfer failure.
2. Transfer Plan Development
- A formal transfer protocol defines acceptance criteria, statistical approaches, and documentation expectations before execution begins.
- Key components include:
- Predefined accuracy and precision acceptance criteria
- Number of replicates for calibration standards and QCs
- Statistical evaluation methods (%Bias, %CV thresholds)
- Deviation handling and root cause documentation plan
- Clear sponsor approval before execution
- This step transforms Bioanalytical Method Transfer from an informal comparison exercise into a regulated, auditable process.
Development challenges reference
3. Execution Phase
- The execution phase generates experimental data to demonstrate method reproducibility in the receiving laboratory.
- Activities typically include:
- Analysis of full calibration curve standards
- QC samples across LLOQ, low, mid, and high levels
- Incurred sample reanalysis (if applicable)
- Evaluation of intra- and inter-run accuracy and precision
- For high-sensitivity assays or complex drug modalities (e.g., biologics, ADCs, oligonucleotides), additional controls may be required to ensure comparability.
For high-sensitivity or complex modalities
4. Statistical Comparison
- Objective statistical analysis confirms whether the transferred method meets predefined equivalence criteria.
- Common statistical tools include:
- %Bias calculations
- %CV assessment
- Linear regression comparison
- Bland–Altman analysis (when appropriate)
- Concordance correlation evaluation
- Increasingly, advanced data analytics and AI-assisted tools support trend analysis and variability detection in modern bioanalysis workflows.
AI-driven improvements in bioanalysis
5. Transfer Report
The transfer report provides documented evidence that the Bioanalytical Method Transfer met regulatory and scientific standards.
The final report includes:
- Raw data review and traceability confirmation
- Audit trail verification
- Deviation and corrective action documentation
- QA review and final approval
- Sponsor sign-off
Regulatory alignment and GLP compliance considerations can be referenced here:
At ResolveMass, our SOP-driven approach ensures every Bioanalytical Method Transfer is inspection-ready.
5: Common Challenges in Bioanalytical Method Transfer
The most common risks in Bioanalytical Method Transfer arise from instrumentation variability, matrix effects, and insufficient documentation.
Major Risk Areas:
- Instrument platform differences
- Analyst technique variability
- Matrix source variability
- Reagent lot changes
- Software version differences
- Incomplete documentation
Stability-related challenges:
https://resolvemass.ca/bioanalytical-stability-testing/
These variables can introduce bias if not proactively controlled.
6: Cross-Validation: When and Why is it required?
Cross-validation is required when two or more laboratories generate bioanalytical data for the same study or when method modifications occur.
Scenarios include:
- Global multi-site clinical trials
- Transition from non-GLP to GLP facility
- Partial method modifications
- Bridging studies
Regulators expect side-by-side analysis of:
- At least 20–30 study samples
- QC samples across ranges
- Statistical comparability
TK and PK-specific considerations:
https://resolvemass.ca/pk-tk-bioanalysis/
https://resolvemass.ca/bioanalytical-cro-services-for-pk-and-tk/
https://resolvemass.ca/toxicokinetic-bioanalysis/
Acceptance criteria typically include ±15–20% difference for most concentrations.
7: Statistical Considerations Bioanalytical Method Transfer
Robust statistical analysis ensures objective demonstration of equivalence.
Common approaches include:
- Paired t-tests
- %Difference calculations
- Deming regression
- Concordance correlation coefficient
Statistical justification strengthens regulatory defensibility and reduces audit findings.
8: Regulatory expectations
Regulators expect pre-defined acceptance criteria, full documentation, and traceable raw data.
Key compliance expectations:
- GLP alignment (if applicable)
- Audit trail review
- Change control documentation
- Deviation justification
- Final QA approval
Regulatory agencies such as the Health Canada and European Medicines Agency emphasize data integrity equally with analytical performance.
9: How early planning reduces risk
Planning Bioanalytical Method Transfer early prevents costly clinical delays.
Best practices:
- Include transfer strategy in IND planning
- Align instrumentation platforms early
- Maintain detailed validation reports
- Archive raw data accessibly
- Conduct pilot transfer runs
Proactive planning transforms transfer from reactive troubleshooting into predictable execution.
10: Bioanalytical Method Transfer for different molecule types
Transfer complexity varies depending on molecule class.
Small Molecules
- Typically LC-MS/MS based
- Matrix interference manageable
- Transfer usually straightforward
- Xenobiotic analysis
Biologics
- Ligand-binding assays
- Reagent variability risk
- Biosimilar support
- Antibody-Drug Conjugate
- Higher inter-lab variability
Oligonucleotides
- Hybridization assays
- Sensitivity challenges
- Extraction efficiency variability
- LC-MS support for oligonucleotides
Cell & Gene Therapies
- Vector quantification
- Complex matrice
Biomarkers & Proteomics
- Biomarker services
- Proteomics support
Each category requires customized Bioanalytical Method Transfer strategies.
11: Why Experience matters?
Experienced laboratories anticipate variability before it becomes a regulatory issue.
At ResolveMass Laboratories Inc., we provide:
- Dedicated transfer protocols
- LC-MS/MS platform harmonization
- QA-reviewed documentation
- Transparent communication with sponsors
- Inspection-ready reporting
ResolveMass supports:
- PK/PD Bioanalysis
- Bioanalytical Services in Drug Development
- Bioanalytical CRO services across phases
Our team combines method development expertise with regulatory compliance knowledge to safeguard your clinical timelines.
12: OUTSOURCING STRATEGY & COST CONSIDERATIONS
Strategic outsourcing reduces operational burden and improves regulatory readiness.
- Bioanalytical Services Overview
- Bioanalytical CRO for Drug Discovery
- Outsource Bioanalysis for Biotech Startups
- Affordable Bioanalytical Services for Biotech Startups
- Cost-Effective Bioanalytical Services
- Bioanalytical Testing Services Cost
- Virtual Bioanalytical Strategy
Conclusion:
Bioanalytical Method Transfer is not merely a laboratory activity—it is a regulatory safeguard protecting the credibility of your PK, TK, and biomarker data.
When executed with structured planning, statistical rigor, and documented compliance, Bioanalytical Method Transfer ensures seamless progression from development to clinical milestones.
For biotechs preparing for IND, CTA, or global clinical expansion, choosing an experienced partner can mean the difference between regulatory confidence and costly delays.
ResolveMass Laboratories Inc. supports biotech innovators with compliant, inspection-ready Bioanalytical Method Transfer and cross-validation strategies tailored to your molecule and development phase.
Frequently Asked Questions:
The primary global guidance is ICH M10 – Bioanalytical Method Validation, issued by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use.
ICH M10 harmonizes expectations across regions including the U.S. Food and Drug Administration, European Medicines Agency, and Health Canada.
It defines requirements for method validation, cross-validation, sample reanalysis, stability testing, and documentation standards to ensure data reliability for regulatory submissions.
Bioanalytical method validation is performed by systematically demonstrating that the method produces accurate, precise, reproducible, and reliable results for its intended use.
The process includes:
-Developing calibration curves
-Running quality control (QC) samples at multiple concentration levels
-Evaluating accuracy and precision across runs
-Assessing selectivity and matrix effects
-Conducting stability studies (bench-top, freeze-thaw, long-term)
-Documenting all results under GLP or regulated conditions
Validation confirms the method is suitable for PK, TK, or biomarker studies.
The commonly recognized 9 validation parameters under regulatory guidance include:
1. Accuracy
2. Precision
3. Selectivity
4. Sensitivity (LLOQ)
5. Linearity / Calibration Curve Performance
6. Recovery
7. Matrix Effect
8. Stability
9. Carryover
Together, these parameters ensure the method consistently quantifies analytes in biological matrices without bias or interference.
The primary purpose of bioanalytical method development is to create a scientifically robust and sensitive method capable of accurately quantifying a drug, metabolite, or biomarker in biological samples.
It ensures:
-Reliable PK/TK profiling
-Dose proportionality assessment
-Safety margin evaluation
-Regulatory-ready data generation
Effective development minimizes later validation failures and supports seamless clinical progression.
In pharmaceutical analysis, analytical methods are broadly categorized into four types:
-Identification Tests : Confirm the identity of an analyte
-Quantitative Tests : Measure concentration or amount
-Limit Tests : Detect impurities at specific thresholds
-Performance Tests : Evaluate functional characteristics (e.g., dissolution)
In bioanalysis, quantitative methods (often LC-MS/MS or ligand-binding assays) are most critical for regulatory submissions.
Reference
- I. Nijem , R. Elliott , J. Brumm , L. Liu , K. Xu , R. Melendez , R. Hendricks , B. Wang , P. Siguenza. Cross validation of pharmacokinetic bioanalytical methods: Experimental and statistical design.https://www.sciencedirect.com/science/article/pii/S0731708524005272
- Howard M Hill &Graeme Smith. Method Transfer Between Bioanalytical Laboratories.https://www.tandfonline.com/doi/full/10.4155/bio.15.37
- Ming Li, Jay Ma, Mark Ma, Meng Chen, James Dalton, Crystal Osei-Tutu. A systematic approach to improve the initial success rates of bioanalytical assay transfers to contract research organizations.https://www.tandfonline.com/doi/abs/10.1080/17576180.2025.2604474
- ICH M10 Bioanalytical Method Validation Guideline-1 year Later.https://link.springer.com/article/10.1208/s12248-024-00974-y

