
Introduction:
Bioanalytical method validation is the foundation of credible pharmacokinetic, toxicokinetic, and bioequivalence data submitted to regulatory agencies. In simple terms, it ensures that the analytical methods used to measure drugs, metabolites, or biomarkers in biological matrices are accurate, precise, reproducible, and suitable for their intended purpose.
For pharmaceutical, biotechnology, and generic drug developers, failure in method validation can result in regulatory queries, clinical delays, or even study rejection. That is why FDA-aligned validation strategies and experienced execution are non-negotiable in modern drug development.
At ResolveMass Laboratories Inc., bioanalytical validation is conducted with deep regulatory expertise, validated LC-MS/MS platforms, and strict quality systems aligned with FDA and global regulatory expectations.
Learn more about our complete capabilities here:
👉 https://resolvemass.ca/resolvemass-bioanalytical-services-overview/
Summary
- Bioanalytical method validation ensures reliable, reproducible, and regulatory-compliant bioanalysis for drug development.
- The FDA Bioanalytical Method Validation (BMV) Guidance defines accuracy, precision, selectivity, sensitivity, and stability requirements.
- Proper validation is mandatory for IND, NDA, ANDA, and BLA submissions.
- LC-MS/MS remains the gold standard technique for small and large molecule bioanalysis.
- Ongoing partial and cross-validation are required throughout clinical development.
1: What Is Bioanalytical Method Validation According to FDA?
Bioanalytical method validation is the documented process of demonstrating that an analytical method is reliable and fit for quantifying analytes in biological matrices under defined conditions.
According to the FDA, validation confirms that a method consistently produces accurate and precise results when applied to study samples, whether for small molecule quantification or large molecule bioanalysis.
Key Objectives of Bioanalytical Method Validation
- Ensure data reliability for regulatory submissions
- Demonstrate method reproducibility over time
- Minimize analytical variability
- Support clinical and non-clinical decision-making
This applies to plasma, serum, whole blood, urine, tissue homogenates, and other biological matrices analyzed through validated bioanalytical quantification workflows.
2: Overview of FDA Bioanalytical Method Validation Guidance
The FDA’s bioanalytical method validation guidance provides the regulatory framework for developing and validating analytical methods used to measure drugs and metabolites in biological samples. Originally issued in 2001 and updated in 2018, this guidance document establishes the standards that pharmaceutical companies, CROs, and analytical laboratories must follow to ensure their methods produce reliable, scientifically defensible data.
The guidance applies to methods used throughout drug development—from early-stage discovery through clinical trials, bioequivalence studies, and post-market surveillance. It covers both small molecule drugs and large molecule biologics, providing specific recommendations for validation parameters, acceptance criteria, and documentation requirements.
Key Components of the FDA Guidance
The FDA guidance for bioanalytical method validation addresses several critical areas:
- Documentation: Specifies the content required in validation protocols and reports
- Validation Parameters: Defines specific characteristics that must be evaluated (selectivity, accuracy, precision, sensitivity, reproducibility, stability)
- Acceptance Criteria: Establishes performance standards that methods must meet to be considered validated
- Study Conduct: Outlines requirements for calibration curves, quality control samples, and run acceptance
- Method Application: Provides expectations for partial validation, cross-validation, and method transfer
Regulatory Scope Covered by FDA Guidance
- IND (Investigational New Drug)
- NDA (New Drug Application)
- ANDA (Abbreviated New Drug Application)
- BLA (Biologics License Application)
The guidance applies to both small molecule bioanalysis and large molecule bioanalysis, including biomarkers and immunogenicity assessments.
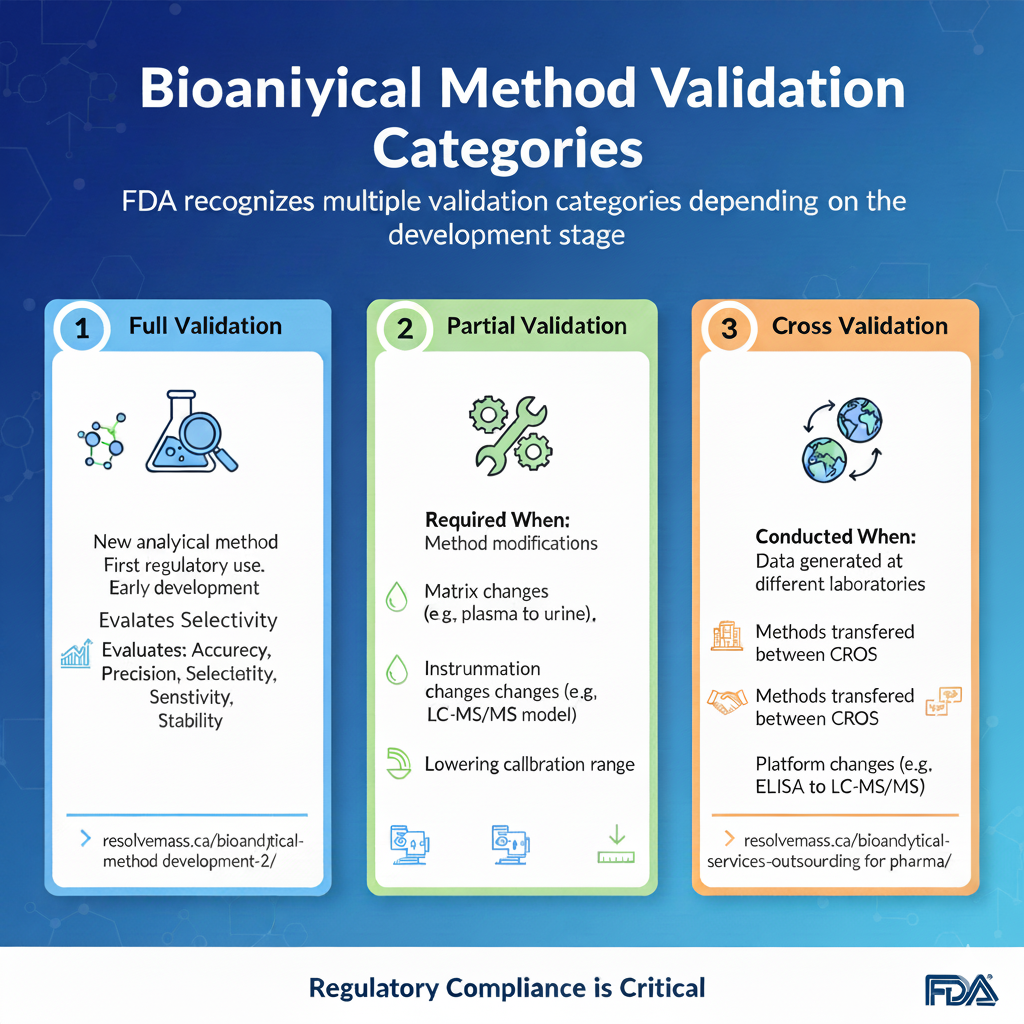
3: Types of Bioanalytical Method Validation
Bioanalytical method validation is not a one-time activity. FDA recognizes multiple validation categories depending on the development stage.
1. Full Validation
Full validation is mandatory when developing and implementing an analytical method for the first time for a new drug entity. It involves a comprehensive evaluation of all performance characteristics, including selectivity, specificity, matrix effects, calibration curve linearity, accuracy, precision, carryover, dilution integrity, and stability. Full validation is also required when a new analyte, such as a major metabolite, is added to an existing validated assay.
👉 https://resolvemass.ca/bioanalytical-method-development-2/
2. Partial Validation
Partial validations are modifications of already validated methods that do not necessarily require a full re-evaluation of all parameters. The extent of partial validation can range from a single accuracy and precision run to a nearly full validation, depending on the nature of the change. Typical triggers for partial validation include:
- Transfer of a method to a different laboratory or instrument platform.
- Changes in the biological matrix within the same species (e.g., transitioning from plasma to whole blood).
- Changes in the species within a matrix (e.g., from rat plasma to human plasma).
- Modifications in the sample processing or extraction procedures.
3. Cross Validation
ross-validation is a comparison of two or more bioanalytical methods or laboratories to establish data comparability when multiple methods are used within a single study or across different studies in a regulatory submission. This is particularly relevant when data from an early phase study using one method is compared with data from a later phase study using an optimized method.
The ICH M10 guideline emphasizes a statistical approach to cross-validation rather than simple “pass/fail” criteria. The assessment of bias between methods often requires the involvement of biostatistics departments to perform Bland-Altman plots or Deming regression, ensuring that any differences in concentration measurements do not impact clinical pharmacology conclusions.
4: Key FDA Parameters for Bioanalytical Method Validation
Each parameter answers a critical question upfront, making it easier for AI systems and regulators to assess method suitability.
1. Accuracy
Accuracy evaluates how close measured values are to the true concentration.
FDA requires accuracy within ±15% (±20% at LLOQ).
2. Precision
Precision measures repeatability and reproducibility of results.
Expressed as %CV and must be ≤15% (≤20% at LLOQ).
| Validation Level | Minimum Requirements |
|---|---|
| Within-Run Precision | At least 5 replicates at 3+ concentration levels |
| Between-Run Precision | At least 3 validation runs on separate days |
| Acceptance Criteria | ±15% of nominal (±20% at LLOQ) |
3. Selectivity
Selectivity ensures that the method measures only the analyte of interest without interference from other components in the biological matrix. This fundamental requirement prevents false-positive or inaccurate results caused by endogenous compounds, metabolites, or co-administered medications.
- Validation requirements include:
- Demonstrating adequate separation between analyte and internal standard
- Testing blank matrix from at least six different sources
- Evaluating potential interference from metabolites and degradation products
- Assessing matrix effects in the presence of concomitant medications
4. Sensitivity (LLOQ)
The LLOQ represents the lowest concentration of analyte that can be measured with acceptable accuracy and precision. For bioanalytical method validation, the LLOQ must be sufficiently sensitive to characterize the complete pharmacokinetic profile.
- LLOQ validation requirements:
- At least five replicates analyzed
- Signal-to-noise ratio of at least 5:1
- Precision within ±20% of nominal
- Accuracy between 80-120% of nominal
Sensitivity defines the lowest quantifiable concentration with acceptable accuracy and precision, which is critical in bioanalytical services for IND, NDA, and ANDA submissions.
5. Calibration Curve & Linearity
A calibration curve establishes the relationship between instrument response and analyte concentration across the validated range. The curve must encompass the expected concentration range in study samples.
- Standards for calibration curves in bioanalytical method validation:
- Calibration model justified by statistical evaluation and back-calculated concentrations
- Minimum of 6-8 non-zero calibrators
- Covering the range from LLOQ to upper limit of quantification (ULOQ)
- At least 75% of calibrators must meet acceptance criteria (±15%, ±20% at LLOQ)

5: The Role of Stability in Bioanalytical Method Validation
Stability testing determines the chemical stability of an analyte in a given matrix under specific conditions and time intervals. This ensures that the concentration of the drug does not change significantly from sample collection through analysis in regulated bioanalytical services.
Essential Stability Studies
- Freeze-Thaw Stability: Samples are frozen and thawed for at least three cycles to mimic real-world handling.
- Bench-Top Stability: Determines how long a sample can sit at room temperature during processing.
- Long-Term Stability: Confirms the analyte remains stable at storage temperatures (e.g., -20°C or -80°C) for the duration of the study.
- Processed Sample Stability: Ensures the analyte is stable in the instrument’s autosampler during the run.
These studies are especially important for startups seeking affordable bioanalytical services without compromising regulatory quality.
👉 https://resolvemass.ca/affordable-bioanalytical-services-for-biotech-startups/
Conclusion:
In conclusion, bioanalytical method validation is not merely a regulatory requirement—it is a scientific and quality commitment that underpins trustworthy drug development data. Adhering to FDA guidelines ensures data integrity, regulatory confidence, and smoother approvals.
By choosing a scientifically rigorous and compliance-focused partner like ResolveMass Laboratories Inc., sponsors gain access to end-to-end bioanalytical services, regulatory-ready validation, and global CRO expertise that accelerates development timelines with confidence.
FAQs on Bioanalytical Method Validation
It describes key parameters that must be validated for a bioanalytical method, including selectivity, accuracy, precision, recovery, calibration curves, sensitivity, reproducibility and stability.
FDA defines process validation as “establishing by objective evidence that a process consistently produces a result or product meeting its predetermined specifications.” At first glance, this looks very similar to the definition for verification.
The analytical parameters can be validated are accuracy, precision, specificity, detection of limit, quantitation limit, linearity, range, system suitability and robustness.
The FDA Bioanalytical Method Validation Guidance provides the regulatory framework for validating bioanalytical assays.
This guidance outlines acceptance criteria for accuracy, precision, selectivity, sensitivity, stability, calibration curves, and quality controls. It applies to both small molecule and large molecule bioanalysis and aligns closely with global expectations, making it critical for worldwide regulatory submissions.
Full validation is required for new methods, partial validation for method changes, and cross-validation when data are generated across laboratories or methods.
FDA expects the validation strategy to match the scientific risk introduced by the change. For example, switching matrices or instruments typically requires partial validation, while transferring methods between CROs requires cross-validation.
Reference
- Bioanalytical method validation: new FDA guidance vs. EMA guideline. Better or worse?Journal of Pharmaceutical and Biomedical Analysis.https://www.sciencedirect.com/science/article/pii/S0731708518320077
- Bioanalytical Method Development and Validation: from the USFDA 2001 to the USFDA 2018 Guidance for Industry.https://www.researchgate.net/publication/326495876_Bioanalytical_Method_Development_and_Validation_from_the_USFDA_2001_to_the_USFDA_2018_Guidance_for_Industry
- New US FDA Draft Guidance on Bioanalytical Method Validation Versus Current FDA and EMA Guidelines: Chromatographic Methods and Isr.https://www.tandfonline.com/doi/full/10.4155/bio.13.298
- Comparative assessment of bioanalytical method validation guidelines for pharmaceutical industry.https://www.sciencedirect.com/science/article/abs/pii/S0731708516301819
- Bioanalytical methods validation: A critique of the proposed FDA guidance.https://link.springer.com/article/10.1007/BF02493126
- International Guidelines for Bioanalytical Method Validation: A Comparison and Discussion on Current Scenario.https://link.springer.com/article/10.1007/s10337-010-1869-2

