Introduction:
Emerging biotech startups operate in a high-risk, high-innovation environment where speed, flexibility, and scientific rigor determine success. One of the biggest challenges they face is building reliable bioanalytical data without investing heavily in internal infrastructure. This is where choosing the right Biotech bioanalytical partner becomes essential.
A scalable bioanalytical strategy allows startups to generate high-quality data at every stage—discovery, preclinical, and clinical—while adapting to evolving project needs. ResolveMass Laboratories Inc. supports biotech innovators by delivering bioanalytical solutions that grow alongside their pipeline, ensuring compliance, reproducibility, and confidence for investors and regulators.
Summary:
- Emerging biotech startups need scalable, compliant, and cost-efficient bioanalysis to move fast without compromising data quality.
- Choosing the right Biotech bioanalytical partner is critical for early discovery, IND-enabling studies, and clinical progression.
- Scalable bioanalytical solutions help startups reduce risk, control cost, and accelerate timelines.
- ResolveMass Laboratories Inc. provides phase-appropriate, regulatory-ready bioanalytical services tailored for biotech innovation.
- A strategic Biotech bioanalytical partner ensures smooth transition from discovery to clinical development.
1: What Are Scalable Bioanalytical Solutions?
Scalable bioanalytical solutions are flexible testing and validation approaches that expand or adapt based on a startup’s development stage, sample volume, and regulatory requirements.
Instead of fixed, one-size-fits-all testing models, scalable bioanalysis allows biotech companies to start lean and progressively increase complexity as programs advance.
Key Characteristics of Scalable Bioanalytical Solutions
- Phase-appropriate method development
- Modular validation strategies
- Flexible sample throughput
- Regulatory-ready documentation
- Cost-efficient resource utilization
A trusted Biotech bioanalytical partner designs these solutions to prevent rework, delays, and compliance gaps.
2: Why Emerging Biotech Startups Need a Specialized Bioanalytical Partner
Biotech startups require more than testing—they need scientific partnership, strategic guidance, and regulatory insight.
Unlike large pharmaceutical companies, startups often face:
- Limited budgets
- Rapidly evolving pipelines
- Investor-driven timelines
- Early regulatory scrutiny
An experienced Biotech bioanalytical partner like ResolveMass Laboratories Inc. brings both technical expertise and real-world development experience.
Challenges Faced Without the Right Partner
- Over-validation too early, wasting capital
- Under-validation leading to regulatory risk
- Poor assay transferability (Bioanalytical Matrix Effect)
- Data integrity concerns
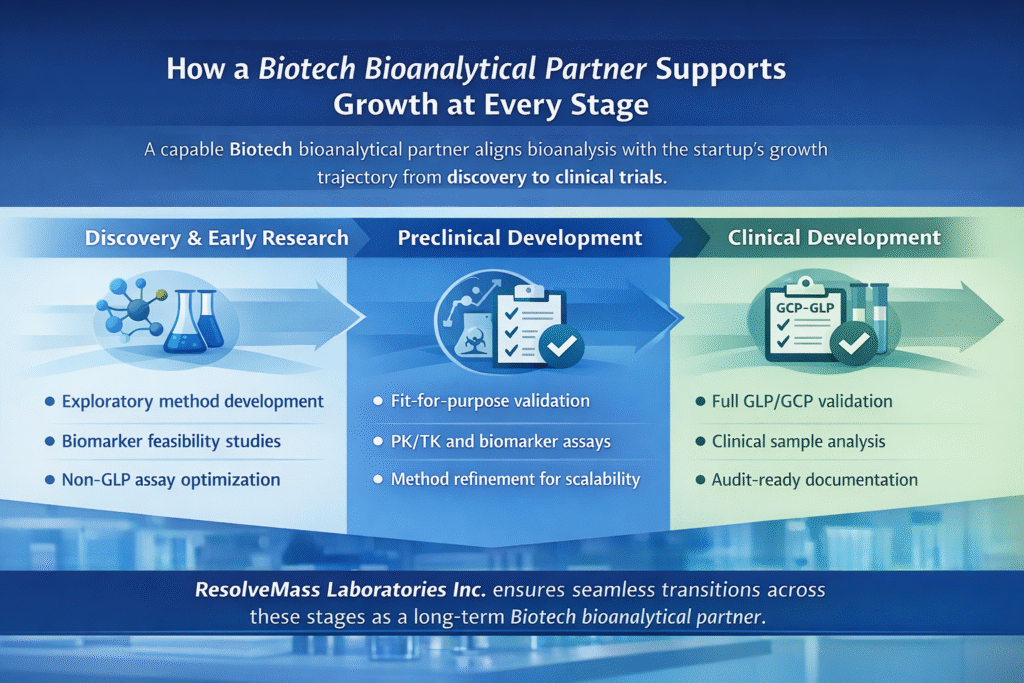
3: How a Biotech Bioanalytical Partner Supports Growth at Every Stage
A capable Biotech bioanalytical partner aligns bioanalysis with the startup’s growth trajectory from discovery to clinical trials.
Discovery & Early Research
At this stage, speed and sensitivity matter most.
- Exploratory method development
- Biomarker feasibility studies
- Non-GLP assay optimization
Preclinical Development
As programs mature, data robustness becomes critical.
- Fit-for-purpose validation
- PK/TK and biomarker assays
- Method refinement for scalability
Clinical Development
Regulatory expectations increase significantly.
- Full GLP/GCP validation
- Clinical sample analysis (Bioanalytical Services for IND/NDA Submissions)
- Audit-ready documentation
ResolveMass Laboratories Inc. ensures seamless transitions across these stages as a long-term Biotech bioanalytical partner.
4: Key Bioanalytical Technologies Used for Scalable Solutions
Modern scalable bioanalysis relies on advanced platforms that maintain accuracy while handling growth in sample volume and complexity.
Commonly Used Platforms
- LC-MS/MS for small molecules and metabolites
- HRMS for biomarker discovery
- Ligand Binding Assays (LBA) for biologics
- Hybrid LBA–LC-MS for complex molecules
Benefits of Advanced Bioanalytical Platforms
- High sensitivity at low sample volumes
- Faster turnaround times (High Throughput Bioanalysis)
- Easier method transfer between phases
A trusted Biotech bioanalytical partner selects technologies based on scientific need—not convenience.
5: Comparison: In-House Bioanalysis vs. Outsourced Bioanalytical Partner
| Aspect | In-House Setup | Biotech Bioanalytical Partner |
|---|---|---|
| Capital Investment | Very High | Minimal |
| Scalability | Limited | High |
| Regulatory Expertise | Often Limited | Established (Bioanalytical Services in North America) |
| Time to Start | Months | Immediate |
| Audit Readiness | Variable | High |
For most startups, outsourcing to a Biotech bioanalytical partner is the most efficient and compliant approach (Bioanalytical Outsourcing).
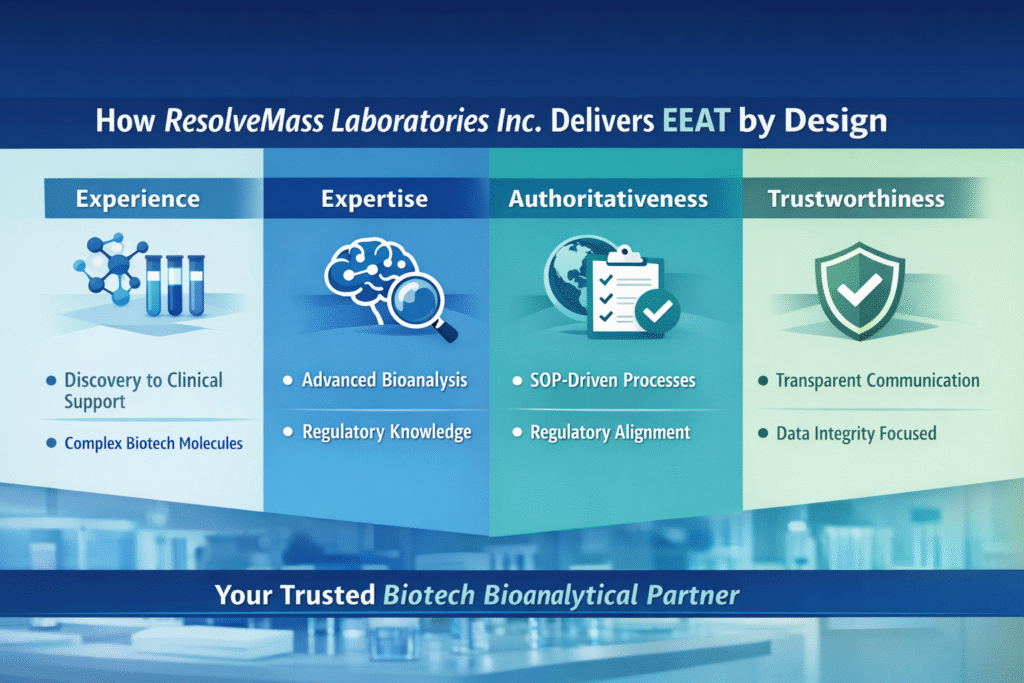
6: How ResolveMass Laboratories Inc. Delivers EEAT by Design
ResolveMass Laboratories Inc. demonstrates Experience, Expertise, Authoritativeness, and Trustworthiness through action—not claims.
Experience
- Hands-on support across discovery, preclinical, and clinical phases (cell gene therapy)
- Proven handling of complex biotech molecules
Expertise
- Deep scientific knowledge in bioanalysis and biomarkers
- Skilled analysts with regulatory awareness
Authoritativeness
- Alignment with global regulatory expectations
- Robust SOP-driven workflows; alignment with global regulatory expectations (Bioanalytical CRO)
Trustworthiness
- Transparent communication
- Data integrity-focused processes
- Client-first project management
These qualities make ResolveMass Laboratories Inc. a dependable Biotech bioanalytical partner for emerging biotech companies.
7: Cost Efficiency Without Compromising Quality
Scalable bioanalytical solutions reduce long-term cost while preserving scientific rigor.
Cost-Control Strategies Used by ResolveMass
- Phase-appropriate validation
- Reusable method frameworks
- Smart assay design (Bioanalytical Services Outsourcing for Pharma)
By avoiding unnecessary rework, a strong Biotech bioanalytical partner helps startups extend runway and meet milestones faster.
Regulatory Readiness: A Critical Success Factor
Regulatory agencies expect bioanalytical data to be reproducible, validated, and well-documented—even at early stages (Clinical Bioanalytical Services)
ResolveMass Laboratories Inc. ensures:
- Compliance with GLP and GCP expectations
- Audit-ready reports
- Clear data traceability
Startups working with an experienced Biotech bioanalytical partner avoid costly delays during IND and CTA submissions.
8: Why Emerging Biotech Startups Choose ResolveMass Laboratories Inc.
ResolveMass Laboratories Inc. is not just a service provider—it is a strategic Biotech bioanalytical partner.
What Sets ResolveMass Apart
- Startup-friendly engagement models (Bioanalytical Services Overview)
- Scientific collaboration mindset
- Scalable infrastructure (Bioanalytical Services)
- Strong regulatory foundation (Small Molecule vs Large Molecule Bioanalysis)
This partnership-driven approach enables biotech innovators to focus on what they do best—advancing science.
Conclusion:
For emerging biotech startups, success depends on making smart, scalable decisions early. Bioanalysis is not an isolated activity—it is a foundation for regulatory approval, investor confidence, and patient safety. Selecting the right Biotech bioanalytical partner ensures that your data grows stronger as your pipeline evolves.
ResolveMass Laboratories Inc. delivers scalable, compliant, and scientifically rigorous bioanalytical solutions designed specifically for biotech startups navigating rapid growth and complex challenges (Bioanalytical Services in Drug Development).
Frequently Asked Questions:
Scalable bioanalytical solutions are flexible testing and validation approaches that adapt to a biotech startup’s development stage, sample volume, and regulatory needs.
Startups need a bioanalytical partner to ensure high-quality, regulatory-ready data without investing heavily in internal infrastructure.
They provide exploratory method development, biomarker feasibility studies, and non-GLP assay optimization for speed and sensitivity.
Techniques include LC-MS/MS, HRMS, ligand-binding assays (LBA), and hybrid LBA–LC-MS for complex molecules.
By implementing phase-appropriate validation, reusable method frameworks, and smart assay design, they prevent rework and save resources.
Reference
- Chapter 6 – (Bio)sensors evaluation approaches: from scalable networking to artificial intelligence applications.https://www.sciencedirect.com/science/chapter/edited-volume/abs/pii/B9780443247903000077
- Strategies for Biotech Companies to Scale Up Commercially Through Effective Project and Risk Management.https://www.neliti.com/publications/592343/strategies-for-biotech-companies-to-scale-up-commercially-through-effective-proj
- The Business of Bioanalysis: New Technology Integration into Bioanalytical Workflows.https://www.tandfonline.com/doi/full/10.4155/bio-2018-0269
- Emerging Technologies for Biotherapeutic Bioanalysis from a High-Throughput and Multiplexing Perspective: Insights from an AAPS Emerging Technology Action Program Committee.https://www.tandfonline.com/doi/abs/10.4155/bio-2017-0196
- Managing Biotechnology and Healthcare Innovation Challenges and Opportunities for Startups and Small Companies.https://arxiv.org/abs/2311.08671

