
Introduction:
Common Bioanalytical Mistakes are one of the most overlooked yet critical causes of delays in drug development programs. Even when a molecule shows strong preclinical promise, errors in bioanalytical method development, validation, sample handling, or documentation can invalidate data and postpone IND submissions. In regulated environments reviewed by agencies such as the U.S. Food and Drug Administration and Health Canada, bioanalytical data must be accurate, reproducible, and fully traceable.
Bioanalysis forms the foundation of pharmacokinetic (PK), toxicokinetic (TK), and exposure-response interpretation. If concentration data are unreliable, dose justification, safety margins, and regulatory decisions become questionable. Many sponsors underestimate why bioanalysis is critical until regulatory review exposes technical gaps.
This article examines the most Common Bioanalytical Mistakes that delay drug development programs and explains how a regulatory-aligned, experience-driven approach can prevent costly setbacks and keep IND timelines on track.
Summary:
- Common Bioanalytical Mistakes often stem from poor method validation and weak quality systems.
- Inconsistent sample handling and stability failures can invalidate entire studies (see stability testing).
- Lack of regulatory alignment leads to data rejection during IND review (IND-enabling insights).
- Poor calibration curve practices and matrix effect evaluation compromise data integrity (matrix effects explained).
- Insufficient communication between CROs and sponsors creates preventable delays (CRO management strategies).
- Early strategic planning and SOP-driven workflows significantly reduce risk.
Drug development timelines are unforgiving. A single analytical oversight can delay an IND submission by months. At ResolveMass Laboratories Inc., we routinely support programs through regulated bioanalytical services designed to withstand agency scrutiny.
1: Why Do Common Bioanalytical Mistakes Delay Drug Development?
Because bioanalytical data directly supports safety, PK/PD interpretation, and regulatory decisions—errors lead to data rejection, repeat studies, and submission delays.
Bioanalytical studies generate concentration-time data that informs:
- Dose selection
- Toxicology interpretation (TK services)
- Exposure-response relationships
- IND-enabling safety margins
If data integrity is compromised, regulators question validation robustness, documentation completeness, and reproducibility.
When Common Bioanalytical Mistakes occur, regulators may question:
- Data integrity
- Method reproducibility
- Validation robustness
- Documentation completeness
In regulated environments, “almost correct” equals “non-compliant.”
2. Inadequate Bioanalytical Method Validation
The most frequent Common Bioanalytical Mistakes involve incomplete or poorly executed validation.
Agencies such as the U.S. Food and Drug Administration and Health Canada require validated methods under formal bioanalytical method validation standards.
Related resources:
Common Validation Gaps
- Insufficient accuracy and precision data
- Poorly defined lower limit of quantification (LLOQ)
- Lack of matrix effect evaluation
- Inadequate recovery assessment
- Selectivity testing across limited matrices
Example Table: Validation Failures & Consequences
| Validation Element | Common Error | Consequence |
|---|---|---|
| Accuracy/Precision | Fewer replicates than required | Regulatory rejection |
| Stability | Incomplete freeze-thaw testing | Re-analysis required |
| Calibration Curve | Poor regression model selection | Biased PK data |
| Matrix Effect | No internal standard normalization | Quantitation errors |
Prevention Strategy:
Develop validation protocols aligned with FDA/Health Canada guidance before first sample analysis.
3. Poor Sample Handling and Stability Assessment
Improper storage, freeze-thaw oversight, or undocumented degradation is a leading contributor to Common Bioanalytical Mistakes.
Comprehensive stability mapping is critical (see: https://resolvemass.ca/bioanalytical-stability-testing/).
For high-sensitivity and advanced LC-MS/MS platforms supporting complex molecules:
Even a fully validated method fails if:
- Plasma samples are stored incorrectly
- Stability is not proven under study conditions
- Hemolysis impact is not assessed
- Freeze-thaw cycles exceed validated limits
Why This Matters
Drug molecules can degrade rapidly. If stability is not demonstrated for:
- Bench-top conditions
- Autosampler storage
- Long-term freezer storage
- Multiple freeze-thaw cycles
Then exposure data becomes scientifically unreliable.
Among all Common Bioanalytical Mistakes, stability oversight is particularly costly because it often affects entire study batches.
4. Calibration Curve and QC Failures
Improper calibration compromises quantitative credibility. These Common Bioanalytical Mistakes often surface during audits.
For robust quantitative strategies
High-throughput analytical workflows reduce variability in large studies
Bioanalytical quantitation relies on calibration curves to convert instrument response into accurate concentration values. If the calibration model is flawed, every reported sample result becomes questionable.
Calibration Curves Must:
- Cover the full expected concentration range of study samples
- Use statistically justified regression models (e.g., weighted 1/x or 1/x² where appropriate)
- Meet acceptance criteria (±15% for standards and QCs, ±20% at LLOQ)
- Include adequate replicate standards across the range
- Demonstrate consistent back-calculated accuracy
Failure in any of these elements can introduce systematic bias into exposure data.
Quality Control (QC) Requirements
QC samples verify that the method performs consistently during sample analysis. Best practices require:
- At least low, mid, and high QC levels
- QCs representing ≥5% of total samples (as per common regulatory expectations)
- Acceptance criteria within ±15% (±20% at LLOQ)
- Distribution of QCs throughout the analytical run
When QC performance trends downward or shows drift, it may indicate instrument instability, matrix effects, or preparation errors.
Typical Calibration and QC Errors
The following Common Bioanalytical Mistakes frequently trigger regulatory concern:
- Over-reliance on forced linear regression without evaluating residual plots
- Ignoring back-calculated standard deviations and systematic bias at curve extremes
- Inadequate weighting model selection, leading to inaccuracy at low concentrations
- Insufficient QC frequency within analytical batches
- Failure to investigate trend failures or partial batch rejections
- Accepting borderline runs without root cause analysis
These errors may appear minor during internal review but become highly visible during inspections by agencies such as the U.S. Food and Drug Administration or Health Canada.
Why This Delays Drug Development
When calibration or QC failures are identified:
- Analytical runs may need to be repeated
- Entire study batches can be rejected
- PK interpretation becomes unreliable
- IND submissions may be delayed
Because calibration data underpin all reported concentrations, regulatory reviewers scrutinize curve fitting, QC performance, and deviation investigations in detail.
Best Practice to Avoid These Common Bioanalytical Mistakes
To prevent calibration-related delays:
- Perform regression model comparison studies during validation
- Use weighted regression where scientifically justified
- Trend QC performance across runs
- Establish clear SOP-driven investigation procedures
- Document all deviations and corrective actions thoroughly
Calibration curves and QC performance are not just technical requirements—they are the mathematical foundation of bioanalytical credibility. Eliminating these Common Bioanalytical Mistakes ensures quantitative reliability, regulatory confidence, and uninterrupted drug development timelines.
5. Matrix Effects and Inadequate Selectivity Testing
Failure to evaluate ion suppression or enhancement biases LC-MS/MS data.
Matrix effect mitigation strategies are detailed.
Advanced strategies for complex drug modalities.
Matrix effects occur when endogenous components suppress or enhance ionization.
Key Oversights
- Limited matrix source testing
- No post-column infusion studies
- Inadequate internal standard selection
- Failure to test lipemic/hemolyzed samples
Experienced bioanalytical laboratories use:
- Stable isotope-labeled internal standards
- Post-extraction addition experiments
- Multiple donor matrices
Ignoring these steps remains one of the more subtle Common Bioanalytical Mistakes that regulators frequently detect.
6. Incomplete Documentation and Audit Trail Weaknesses
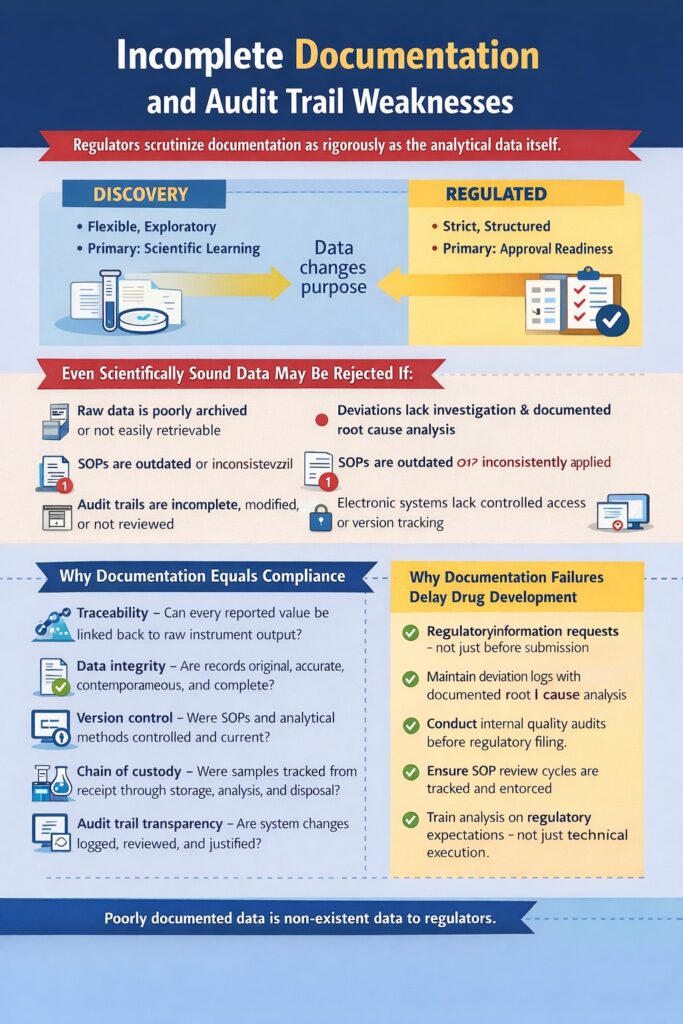
Incomplete documentation and weak audit trails are among the most serious Common Bioanalytical Mistakes because regulators evaluate documentation as rigorously as analytical performance. Even scientifically sound, technically accurate data can be rejected if supporting records are inconsistent, incomplete, or non-traceable.
Documentation failures are among the most preventable Common Bioanalytical Mistakes.
For regulated laboratory systems.
Discovery vs regulated environments explained.
In regulatory submissions reviewed by agencies such as the U.S. Food and Drug Administration and Health Canada, documentation is not considered administrative paperwork—it is proof of data integrity.
Even Scientifically Sound Data May Be Rejected If:
- Raw data is poorly archived or not easily retrievable
- Deviations lack proper investigation and root cause analysis
- Standard Operating Procedures (SOPs) are outdated or inconsistently applied
- Audit trails are incomplete, modified, or not reviewed
- Electronic systems lack controlled access or version tracking
Among all Common Bioanalytical Mistakes, documentation failures are particularly dangerous because they may not become apparent until a regulatory audit or submission review.
Why Documentation Equals Compliance
Regulatory reviewers assess whether data can be reconstructed step-by-step from sample receipt to final report. If traceability breaks at any point, confidence in the dataset declines.
Regulatory Reviewers Evaluate:
- Traceability – Can every reported value be linked back to raw instrument output?
- Data integrity – Are records original, accurate, contemporaneous, and complete?
- Version control – Were SOPs and analytical methods controlled and current?
- Chain of custody – Were samples tracked from receipt through storage, analysis, and disposal?
- Audit trail transparency – Are system changes logged, reviewed, and justified?
If any of these elements are missing, regulators may question whether data manipulation, procedural drift, or systemic weakness occurred—even when none actually did.
Why Documentation Failures Delay Drug Development
Documentation-related Common Bioanalytical Mistakes can result in:
- Regulatory information requests (IRs)
- Data reanalysis or reprocessing
- On-site inspections
- IND submission delays
- Loss of sponsor confidence
Unlike technical assay optimization, documentation control does not require new instrumentation—only disciplined quality systems and regulatory awareness. That is why documentation failures are the most preventable category of bioanalytical delay.
Best Practices to Prevent Documentation Weaknesses
- Implement controlled electronic data management systems
- Review audit trails routinely, not just before submission
- Maintain deviation logs with documented root cause analysis
- Conduct internal quality audits before regulatory filing
- Ensure SOP review cycles are tracked and enforced
- Train analysts on regulatory expectations—not just technical execution
In bioanalysis, data without documentation is considered non-existent from a regulatory perspective. Eliminating documentation-related Common Bioanalytical Mistakes ensures that analytical excellence is supported by compliance, credibility, and audit readiness—protecting both your submission timeline and your scientific integrity.
7. Lack of Regulatory Alignment in Early Development
Failure to align bioanalytical strategy with regulatory expectations is one of the most costly Common Bioanalytical Mistakes, often leading to IND delays or additional data requests. When early development decisions are made without regulatory foresight, programs frequently require revalidation, bridging studies, or repeated analyses before submission.
Regulatory misalignment often forces bridging studies or partial revalidation before IND submission.
- For submission-ready support: https://resolvemass.ca/bioanalytical-services-for-ind-nda-submissions/
- Comprehensive drug development bioanalysis: https://resolvemass.ca/bioanalytical-services-in-drug-development/
- Virtual strategy planning for early biotech: https://resolvemass.ca/virtual-bioanalytical-strategy/
Bioanalytical data submitted to agencies such as the U.S. Food and Drug Administration and Health Canada must demonstrate not only scientific accuracy but also regulatory compliance. If alignment is missing from the beginning, corrections later become expensive and time-consuming.
Early-Stage Companies Often:
- Skip partial validation steps, assuming full validation can wait
- Underestimate bridging requirements when modifying methods or matrices
- Fail to document method transfers between labs or analytical platforms
- Ignore cross-validation requirements for multi-site studies
- Overlook regulatory reporting format expectations
These Common Bioanalytical Mistakes typically arise from prioritizing speed over regulatory strategy. While this may accelerate early research milestones, it frequently creates bottlenecks at the IND stage.
Why Early Regulatory Alignment Matters
Bioanalytical strategy should evolve alongside clinical and toxicology planning. Without regulatory alignment:
- Method changes require bridging studies
- Platform transfers demand cross-validation
- Matrix modifications require partial revalidation
- Agencies may issue information requests (IRs)
Each corrective step adds weeks—or months—to development timelines.
What a Regulatory-Aligned Bioanalytical Plan Includes
A structured, forward-looking plan reduces risk significantly.
1. Pre-IND Strategy Discussions
- Define submission goals early
- Align assay sensitivity with expected exposure levels
- Anticipate agency expectations
2. Defined Acceptance Criteria
- Pre-established validation thresholds
- Clearly documented run acceptance rules
- Transparent deviation handling procedures
3. Risk-Based Validation
- Evaluate molecule stability and matrix complexity
- Adjust validation scope based on intended use
- Document justification for validation design
4. Clearly Documented SOP Framework
- Controlled method versioning
- Documented method transfers
- Structured cross-validation procedures
- Audit-ready workflows
How This Prevents Common Bioanalytical Mistakes
Regulatory alignment ensures that analytical work performed today remains defensible at submission. Instead of reactive corrections, development teams operate with:
- Predictable validation pathways
- Reduced need for repeat studies
- Clear regulatory communication
- Higher confidence during review
Among all Common Bioanalytical Mistakes, lack of early regulatory alignment is particularly damaging because it affects the entire development lifecycle—not just a single study.
Proactive, regulatory-driven bioanalytical planning protects IND timelines, preserves investor confidence, and ensures that strong science translates into successful regulatory progression.

8. Poor Communication Between Sponsor and CRO
Misalignment between sponsor and CRO teams leads to preventable delays and rework.
Typical communication gaps:
- Undefined sample timelines
- Incomplete method transfer documentation
- Lack of clarity on regulatory submission plans
- Inconsistent reporting formats
An experienced CRO proactively asks:
- What submission is this data supporting?
- Is this GLP or non-GLP?
- What agency review is anticipated?
Avoiding these Common Bioanalytical Mistakes requires technical expertise and structured project management.
Outsourcing bioanalysis requires structured oversight.
Resources:
- Bioanalytical CRO
- CRO for Drug Discovery
- Outsourced Bioanalysis
- Outsourced Drug Development Bioanalysis
- Bioanalytical CRO for PK & TK
Cost considerations for startups:
https://resolvemass.ca/affordable-bioanalytical-services-for-biotech-startups/
https://resolvemass.ca/cost-effective-bioanalytical-services/
https://resolvemass.ca/bioanalytical-testing-services-cost/
9. Ignoring Bioanalytical Risk Assessment
Programs often proceed without formal analytical risk assessment, increasing failure probability.
A risk-based framework evaluates:
- Molecule stability
- Sensitivity challenges
- Matrix variability
- Analytical selectivity
Early identification of analytical challenges reduces downstream delays.
A structured risk-based framework reduces failure probability.
Specialized modalities require tailored expertise:
- Oligonucleotide Bioanalysis
- Antibody-Drug Conjugates
- Cell & Gene Therapy Bioanalysis
- Biosimilar Bioanalysis
- Biomarker Bioanalytical Services
- Proteomics Services
AI-driven approaches in bioanalysis
10: How ResolveMass Laboratories Inc. Prevents These Common Bioanalytical Mistakes
At ResolveMass Laboratories Inc., our approach is built on:
1. Regulatory-Driven Method Development
- Designed from day one to meet FDA and Health Canada expectations
- Clear validation protocols
2. SOP-Based Analytical Execution
- Controlled documentation systems
- Audit-ready workflows
3. Stability-Centered Planning
- Full stability mapping before pivotal studies
- Real-time tracking of storage conditions
4. Transparent Client Communication
- Defined timelines
- Regular technical updates
- Clear data reporting structure
Our team combines practical laboratory experience with regulatory strategy—reducing risk across IND-enabling programs.
Our integrated capabilities include:
- Full Bioanalytical Services Overview
- Clinical Bioanalytical Services
- PK/PD Bioanalysis
- PK/TK Bioanalysis
- Complete Bioanalytical Services Portfolio
Our regulatory-driven, SOP-controlled framework ensures analytical data withstands scrutiny and supports confident IND progression.
Quick Checklist: Avoiding Common Bioanalytical Mistakes
Before starting your next study, confirm:
- ✔ Validation protocol approved
- ✔ Stability conditions fully mapped
- ✔ Calibration model justified
- ✔ Matrix effects evaluated across donors
- ✔ QC acceptance criteria defined
- ✔ Documentation audit trail verified
- ✔ Regulatory alignment confirmed
10: The Financial Impact of Bioanalytical Delays
Bioanalytical errors don’t just delay timelines—they increase costs:
- Repeat animal studies
- Additional CRO contracts
- Delayed investor milestones
- Extended burn rates
Addressing Common Bioanalytical Mistakes early protects both scientific credibility and financial runway.
Conclusion:
Common Bioanalytical Mistakes are not minor technical oversights—they directly impact regulatory approval timelines, investor confidence, and program viability.
From incomplete validation to documentation gaps and matrix effect oversight, these mistakes can delay drug development programs significantly.
The solution is not reactive troubleshooting—but proactive, regulatory-aligned bioanalytical strategy.
If your IND-enabling study depends on defensible, audit-ready bioanalytical data, our experts at ResolveMass Laboratories Inc. are ready to support your program.
Frequently Asked Questions:
Common Bioanalytical Mistakes include incomplete audit trails, missing raw data, outdated SOPs, and poor version control. Laboratories sometimes fail to document deviations or investigations properly. Inconsistent sample tracking is another frequent issue. These gaps often remain unnoticed until regulatory review. Most problems arise from weak quality systems rather than scientific limitations.
Regulators evaluate both analytical performance and data integrity. Even accurate results can be questioned if documentation is incomplete. If raw data cannot be reconstructed step-by-step, confidence is lost. Missing audit trails or unclear data processing steps raise red flags. Regulatory acceptance depends on transparency, traceability, and compliance.
Regulators examine traceability from raw data to final reports. They review audit trails, system access controls, and SOP version history. Deviation investigations and corrective actions are carefully assessed. Inspectors also check whether method validation meets guidance standards. The goal is to confirm data integrity and regulatory alignment.
Weak audit trails can trigger additional information requests. Regulators may ask for data reconstruction or reanalysis. This increases review timelines and operational burden. In serious cases, it may require re-validation work. Strong audit trail governance prevents unnecessary submission delays.
Most documentation issues stem from process weaknesses, not scientific challenges. Clear procedures and defined responsibilities reduce inconsistencies. Digital systems with proper access controls improve traceability. Proactive quality culture minimizes oversight gaps. With planning and discipline, Common Bioanalytical Mistakes can be avoided before submission.
Reference
- Saurabh Pandey , Preeti Pandey , Gaurav Tiwari , Ruchi Tiwari. Bioanalysis in drug discovery and development.https://www.sciencedirect.com/science/article/abs/pii/S2229470810110036
- Applicability of bioanalysis of multiple analytes in drug discovery and development: review of select case studies including assay development considerations.https://analyticalsciencejournals.onlinelibrary.wiley.com/doi/abs/10.1002/bmc.594
- Brigitte Buscher, Sirpa Laakso , Hermann Mascher , Klaus Pusecker , Mira Doig, Lieve Dillen. Bioanalysis for Plasma Protein Binding Studies in Drug Discovery and Drug Development: Views and Recommendations of The European Bioanalysis Forum.https://www.tandfonline.com/doi/full/10.4155/bio.13.338
- Biopharmaceutical analysis — current analytical challenges, limitations, and perspectives.https://link.springer.com/article/10.1007/s00216-025-06036-2

