INTRODUCTION: WHY CUSTOM API SYNTHESIS MATTERS
Custom API Synthesis has become a foundational requirement in modern pharmaceutical development, especially for generic and specialty drug manufacturers. As regulatory expectations tighten and molecular complexity increases, relying on standard or catalog APIs often leads to quality, compliance, and scalability challenges.
In today’s competitive landscape, Custom API Synthesis allows pharmaceutical companies to maintain control over chemistry, impurities, cost, and regulatory strategy—making it essential for successful product development and commercialization.
The pharmaceutical industry is rapidly evolving, with increasing demand for high-quality Active Pharmaceutical Ingredients (APIs) tailored for both generic and specialty drugs. Custom API synthesis plays a crucial role in meeting these demands by offering precise, high purity, and scalable API production. It enables pharmaceutical companies to develop cost-effective generics and innovate in specialty drug formulations, ensuring therapeutic efficacy, regulatory compliance, and market competitiveness.
IIn this blog, we explore the significance of custom API synthesis for generic and specialty pharmaceuticals, its advantages, challenges, and the future trends shaping this industry.
SUMMARY – KEY TAKEAWAYS
- Custom API Synthesis enables pharmaceutical companies to design APIs that meet specific regulatory, quality, and cost requirements.
- It is critical for generic drug manufacturers to overcome patent constraints and control impurity profiles.
- Specialty pharmaceuticals rely on custom synthesis to manage complex chemistries and high-potency APIs.
- Custom API Synthesis improves scalability, supply chain security, and long-term commercial viability.
- Partnering with an experienced laboratory like ResolveMass Laboratories Inc. ensures scientific rigor, regulatory readiness, and trust.
1: WHAT IS CUSTOM API SYNTHESIS?
Custom API Synthesis is the tailored development and manufacturing of active pharmaceutical ingredients based on specific client and project requirements. Unlike off-the-shelf APIs, custom synthesis involves designing unique synthetic routes, impurity control strategies, and scalable processes aligned with regulatory submissions.
Key elements of Custom API Synthesis include:
- Custom route scouting and process optimization
- Controlled impurity and residual solvent profiles
- Scalability from milligram to multi-kilogram levels
- Compliance with ICH, FDA, EMA, and global guidelines
Custom API Synthesis ensures the API is chemically, physically, and regulatory fit for its intended use.
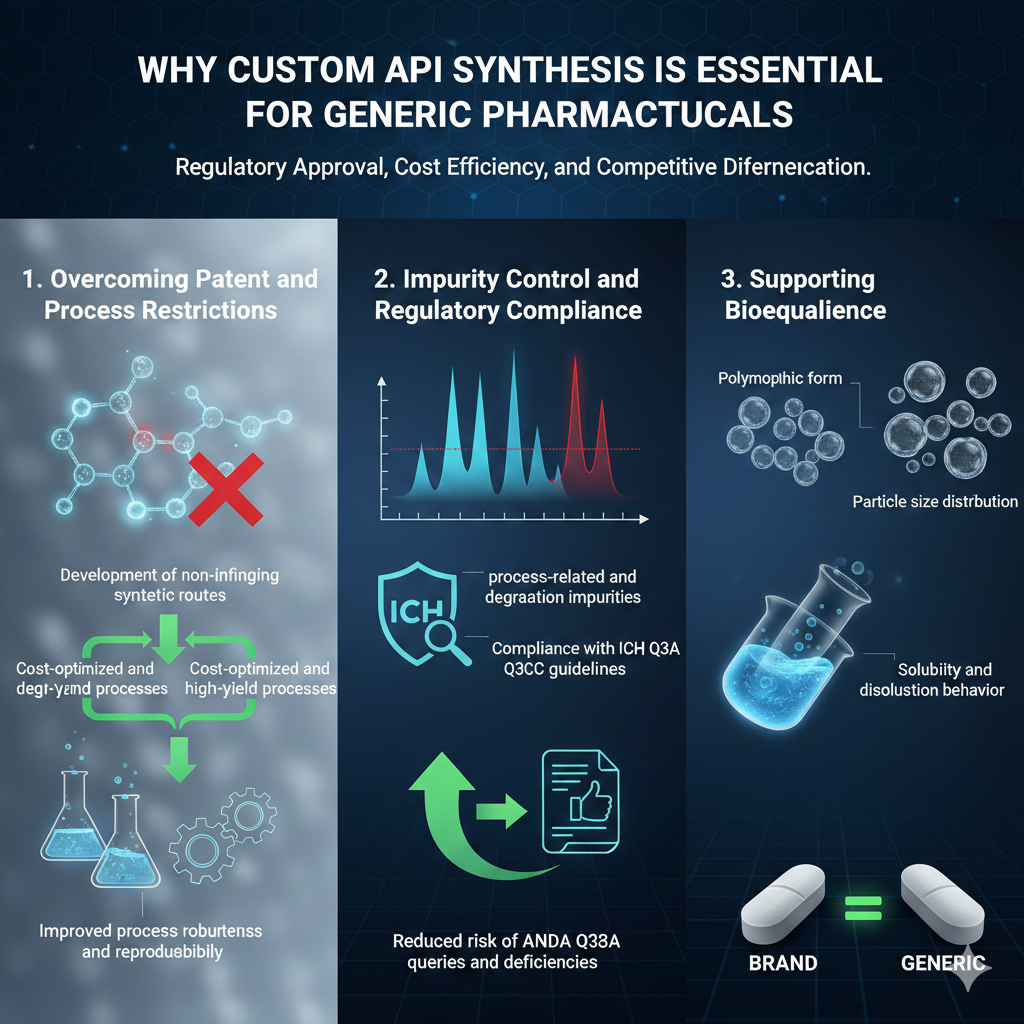
2: WHY CUSTOM API SYNTHESIS IS ESSENTIAL FOR GENERIC PHARMACEUTICALS
Custom API Synthesis is essential for generic pharmaceuticals to ensure regulatory approval, cost efficiency, and competitive differentiation.
1. Overcoming Patent and Process Restrictions
Generic manufacturers cannot always replicate innovator synthesis routes due to intellectual property barriers. Custom API Synthesis enables:
- Development of non-infringing synthetic routes
- Cost-optimized and high-yield processes
- Improved process robustness and reproducibility
2. Impurity Control and Regulatory Compliance
Regulatory authorities place significant emphasis on impurity profiling. Custom API Synthesis supports:
- Identification of process-related and degradation impurities
- Compliance with ICH Q3A and Q3C guidelines
- Reduced risk of ANDA queries and deficiencies
3. Supporting Bioequivalence
API characteristics directly influence bioequivalence outcomes. Through Custom API Synthesis, manufacturers can control:
- Particle size distribution
- Polymorphic form
- Solubility and dissolution behavior
3: Understanding Custom API Synthesis
Custom API synthesis refers to the process of developing and producing APIs with specific molecular characteristics, purity levels, and synthesis routes tailored to a pharmaceutical company’s unique requirements. This process is particularly vital for:
- Generic pharmaceuticals, where APIs must be identical in composition and efficacy to branded drugs.
- Specialty pharmaceuticals, where APIs are customized for complex, rare, or personalized therapies.
Custom API synthesis involves multi-step organic synthesis, process optimization, and stringent analytical validations to ensure that APIs meet global regulatory standards such as those set by the FDA, EMA, and ICH.
4: The Importance of Custom API Synthesis in Generic Pharmaceuticals
1. Cost-Effective Drug Development
Generic drug manufacturers rely on custom API synthesis to develop cost-effective alternatives to branded medications. With patent expirations of blockbuster drugs, pharmaceutical companies seek high-quality APIs at a competitive price to meet market demand.
2. Regulatory Compliance and Quality Assurance
Regulatory bodies such as the FDA and EMA enforce stringent guidelines for generics to ensure bioequivalence and safety. Custom API synthesis ensures that APIs meet these rigorous standards through Good Manufacturing Practices (GMP), ICH Q7 compliance, and analytical validations 1.
3. Scalable and Consistent Production
API synthesis provides a robust and scalable production platform, ensuring batch-to-batch consistency and reproducibility. This is essential for maintaining therapeutic efficacy and minimizing variations in drug formulations.
APIs do the work, but polymers deliver the results – The Importance of Polymer Synthesis in Modern Science and Technology explains their role in modern pharma.

🧪 APIs Do the Work – Polymers Deliver the Results
APIs do the work, but polymers deliver the results – this guide explains their role in modern pharma. .
👉 Explore how polymers are transforming pharma »5: The Role of Custom API Synthesis in Specialty Pharmaceuticals
Custom API Synthesis is indispensable for specialty pharmaceuticals due to complex molecular structures and stringent quality expectations.
Managing Complex Chemistry
Specialty APIs often involve:
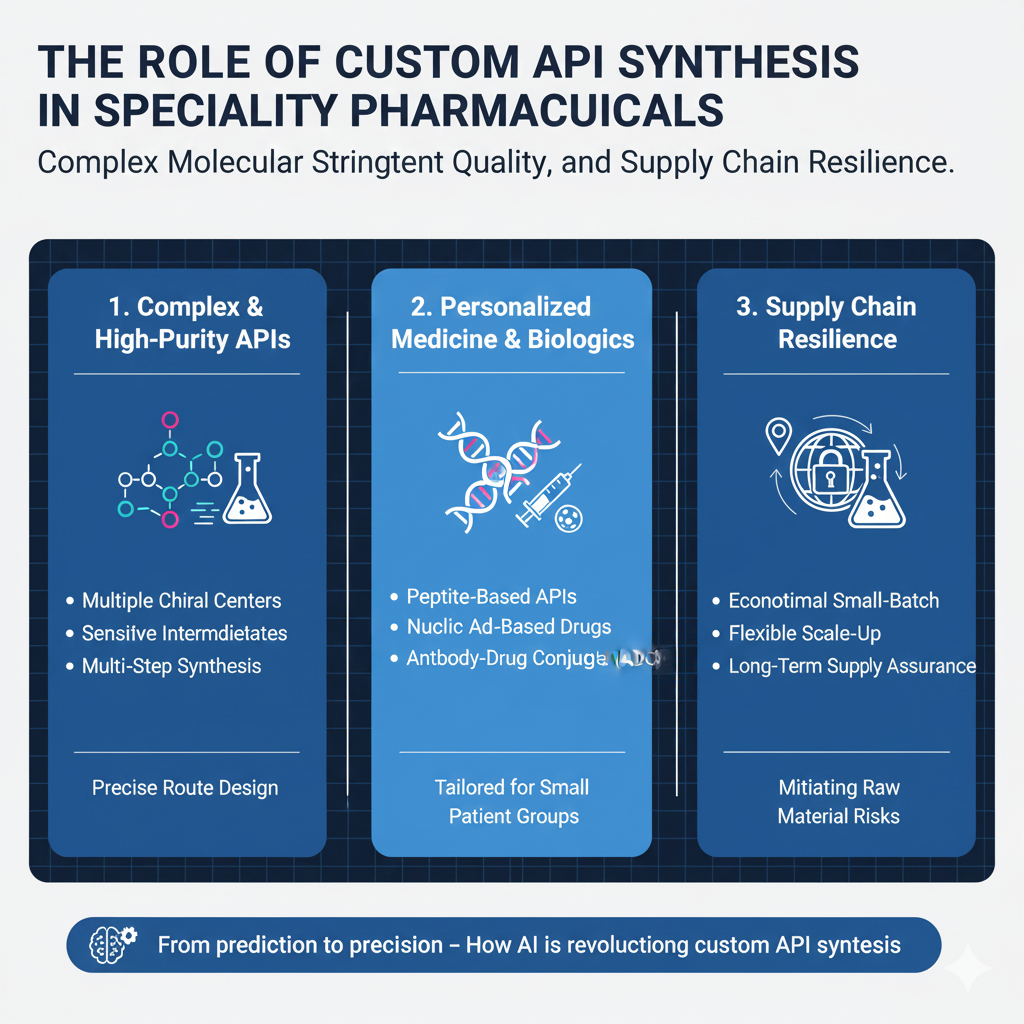
- Multiple chiral centers
- Sensitive intermediates
- Multi-step synthesis pathways
Custom API Synthesis allows precise route design and advanced analytical monitoring to ensure consistent quality.
High-Potency and Low-Dose APIs
Many specialty drugs require extremely low doses. Custom synthesis enables:
- Tight impurity control at trace levels
- Enhanced safety and containment strategies
- Reliable batch-to-batch consistency
Low-Volume and Orphan Drug Support
Commercial API suppliers may not support small or niche volumes. Custom API Synthesis provides:
- Economical small-batch production
- Flexible scale-up options
- Long-term supply assurance
1. Development of Complex and High-Purity APIs
Specialty pharmaceuticals often require APIs with unique molecular architectures, stereochemical precision, and high purity. Custom synthesis enables the development of these APIs with precision and efficiency.
2. Personalized Medicine and Biologics
Advancements in personalized medicine and biologics demand APIs tailored for individual patients or small patient groups. Peptide-based APIs, nucleic acid-based drugs, and antibody-drug conjugates (ADCs) are some examples where custom synthesis is crucial.
From prediction to precision – see how AI is helping scientists build better APIs faster How AI Is Revolutionizing Custom Polymer Synthesis
3. Overcoming Supply Chain Challenges
Global supply chain disruptions highlight the need for localized and flexible API synthesis. Custom API manufacturing ensures reliable supply, reducing dependence on overseas sources and mitigating risks associated with raw material shortages.
6: Key Challenges in Custom API Synthesis
1. Complex Synthesis Pathways
Many specialty APIs require multi-step synthesis with intricate reaction mechanisms, making scale-up and cost-efficiency a challenge.
2. Regulatory Hurdles
Ensuring regulatory approval for new APIs involves extensive documentation, analytical validations, and compliance with evolving global standards 3.
3. Environmental and Safety Considerations
Stringent environmental regulations mandate the use of green chemistry principles and process optimization to minimize hazardous waste and energy consumption.
CUSTOM API SYNTHESIS VS. CATALOG API
| Parameter | Custom API Synthesis | Catalog API |
|---|---|---|
| Process Flexibility | High | Limited |
| Impurity Control | Customized | Standard |
| Regulatory Alignment | Product-specific | Generic |
| Scalability | Planned | Often constrained |
| Cost Optimization | Strategic | Fixed |
7: Future Trends in Custom API Synthesis
1. Adoption of Green Chemistry
Sustainable API synthesis through catalysis, biocatalysis, and flow chemistry is gaining traction to minimize environmental impact and enhance efficiency.
2. Artificial Intelligence (AI) in API Development
AI and machine learning-driven process optimization are revolutionizing API synthesis by predicting reaction outcomes and streamlining process development.
3. Increased Focus on Continuous Manufacturing
Continuous flow manufacturing is replacing traditional batch processes, offering enhanced control, scalability, and reduced production time.
Custom API Synthesis will continue to grow in importance as pharmaceutical pipelines become more complex.
Emerging trends include:
- Increased adoption of green and sustainable chemistry
- Greater integration of analytics with process development
- Higher regulatory expectations for impurity knowledge
Companies investing in advanced Custom API Synthesis strategies will gain long-term competitive advantages.
Conclusion
API synthesis is an indispensable part of modern pharmaceutical development, enabling the production of high-quality, cost-effective generic drugs and innovative specialty pharmaceuticals. By integrating advanced synthetic methodologies, regulatory compliance, and sustainable practices, pharmaceutical companies can leverage custom API synthesis to gain a competitive edge in the global market.
Custom API Synthesis is no longer optional for generic and specialty pharmaceutical companies. It is a strategic necessity that ensures control over chemistry, quality, cost, and compliance. By enabling tailored development and regulatory-ready APIs, Custom API Synthesis directly impacts product success and market sustainability.
Partner with ResolveMass Laboratories Inc. to ensure your Custom API Synthesis program is scientifically sound, compliant, and future-ready.
Global Market Insights: The Future of Custom Polymer Synthesis – click here to understand how global market trends are shaping the future of custom chemical prediction.
REFERENCES
- Chaudhuri A. Simultaneous improvement in development time, cost and quality: a practical framework for generic pharmaceuticals industry. R&d Management. 2013 Jun;43(3):227-41.
- Burke AJ, Marques CS, Turner NJ, Hermann GJ. Active pharmaceutical ingredients in synthesis: catalytic processes in research and development. John Wiley & Sons; 2018 Jul 20.
- Pollak P, Badrot A, Dach R. API manufacturing: facts and fiction. Contract Pharma. 2012;14(1):56-66.
How to Build a Virtual Chemistry Department Using an Outsourced CRO Model
Summary Building a Virtual Chemistry Department through an outsourced CRO model allows biotech and pharma…
Drug Discovery CRO Chemistry Services: What Early-Stage Biotechs Should Expect
🔍 Summary at a Glance Clear deliverables, timelines, and communication protocols are critical when outsourcing…
End-to-End Biomarker Bioanalytical Services Offered by a CRO: Capabilities and Expectations
Introduction Biomarker bioanalytical services CRO partnerships have become indispensable in modern pharmaceutical development, providing the…
Regulatory-Compliant Biomarker Bioanalytical Services for FDA and Health Canada Submissions
Introduction Biomarker bioanalytical services for FDA and Health Canada submissions require rigorous compliance with regulatory…
Outsourced Medicinal Chemistry for Startups: Cost, Speed, and Scientific Advantages
🔍 Summary (Key Takeaways) Outsourced medicinal chemistry for startups offers a strategic advantage by minimizing…
Why Early-Stage Biotech Companies Outsource Chemistry Services to Drug Discovery CROs
Introduction Early-stage biotech companies are increasingly adopting Outsourced Chemistry Services for Biotech to stay competitive…