Introduction:
Data Integrity in Bioanalytical studies is the foundation of regulatory trust, patient safety, and successful drug development. Without reliable and traceable data, even scientifically sound results can be rejected during regulatory review.
As pharmaceutical and biotech companies increasingly outsource PK, TK, biomarker, and immunogenicity testing, ensuring Data Integrity in Bioanalytical studies becomes both a scientific and compliance challenge.
ResolveMass Laboratories Inc. supports sponsors through comprehensive
- 👉 https://resolvemass.ca/bioanalytical-services/
- 👉 https://resolvemass.ca/bioanalytical-services-outsourcing-for-pharma/
- 👉 https://resolvemass.ca/bioanalytical-cro/
Regulators now scrutinize documentation, audit trails, electronic systems, and analyst practices as closely as analytical performance itself.
Summary:
- Data Integrity in Bioanalytical studies is critical for regulatory acceptance, IND/NDA approvals, and long-term program credibility.
- Outsourcing increases complexity, making sponsor oversight and CRO qualification essential.
- Regulatory agencies such as the U.S. Food and Drug Administration, Health Canada, and European Medicines Agency expect complete, attributable, legible, contemporaneous, original, and accurate (ALCOA+) data.
- Audit trails, validated computerized systems, and documented SOPs are non-negotiable.
- Proactive quality systems and sponsor–CRO transparency reduce inspection findings and data rejection risks.
- Selecting an experienced partner like ResolveMass Laboratories Inc. strengthens compliance and scientific defensibility.
1: What Is Data Integrity in Bioanalytical Studies?
Data Integrity in Bioanalytical studies means that all generated data are complete, consistent, accurate, and reliable throughout the entire study lifecycle — from sample receipt to final regulatory submission. It ensures that reported results truly reflect what was performed in the laboratory and can withstand regulatory scrutiny.
Regulatory authorities such as the U.S. Food and Drug Administration and European Medicines Agency evaluate bioanalytical data using the ALCOA+ principles.
ALCOA+ Principles
Data must be:
- Attributable – Clearly linked to the individual who generated or modified it
- Legible – Readable and permanently recorded
- Contemporaneous – Documented at the time the activity occurred
- Original – Preserved in its first recorded form or as a certified true copy
- Accurate – Free from errors and scientifically correct
- Complete, Consistent, Enduring, and Available – Maintained without gaps, protected from loss, and accessible for review
Scope in Outsourced Bioanalytical Studies
In outsourced environments, Data Integrity in Bioanalytical studies extends beyond analytical testing and includes:
- Sample chain-of-custody documentation
- Electronic laboratory information management systems (LIMS)
- Chromatography and mass spectrometry data systems
- Method validation records and raw data
- Audit trails and user access controls
- Statistical analysis outputs and reporting files
Strong data integrity ensures regulatory confidence, protects patient safety, and safeguards the credibility of pharmacokinetic, toxicokinetic, and biomarker study outcomes.
Agencies including the U.S. Food and Drug Administration and European Medicines Agency routinely issue warning letters citing:
- Deleted chromatographic runs
- Incomplete audit trails
- Uncontrolled spreadsheet calculations
- Backdated entries
- Missing raw data
For IND, NDA, ANDA, and BLA submissions, bioanalytical data directly support:
- Dose selection
- Safety margins
- Exposure-response relationships
- Toxicokinetic justification
If Data Integrity in Bioanalytical studies is compromised, regulatory reviewers may:
- Request reanalysis
- Issue clinical holds
- Delay approvals
- Reject applications
Related services supporting compliant programs:
- https://resolvemass.ca/bioanalytical-method-validation/
- https://resolvemass.ca/bioanalytical-method-development-2/
- https://resolvemass.ca/rapid-bioanalytical-method-development/
- https://resolvemass.ca/bioanalytical-quantification/
- https://resolvemass.ca/bioanalytical-matrix-effects/
- https://resolvemass.ca/challenges-in-bioanalytical-method-development/
For regulated submissions:
- https://resolvemass.ca/ind-enabling-bioanalytical-studies/
- https://resolvemass.ca/bioanalytical-services-for-ind-nda-submissions/
- https://resolvemass.ca/bioanalytical-services-in-drug-development/
If Data Integrity in Bioanalytical studies is compromised, regulators may request reanalysis, issue clinical holds, delay approvals, or reject submissions.
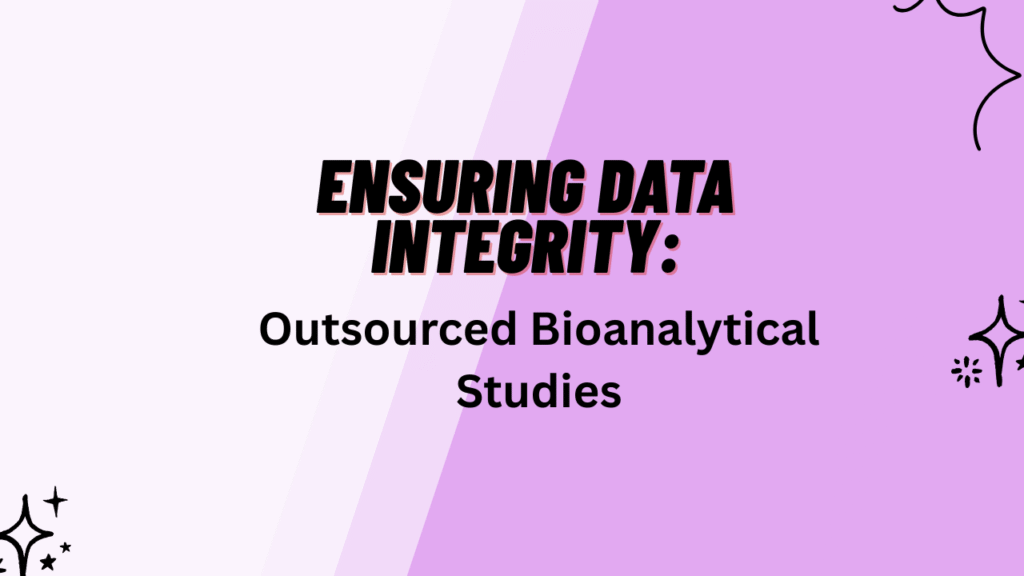
2: Common Data Integrity Risks in Outsourced Bioanalytical Studies
The most frequent risks in Data Integrity in Bioanalytical studies arise from weak sponsor oversight, poorly controlled electronic systems, and inadequate documentation practices. In outsourced environments, these risks increase because sponsors rely on third-party laboratories for both scientific execution and data governance.
Regulatory authorities such as the U.S. Food and Drug Administration and European Medicines Agency routinely cite these deficiencies during inspections.
For deeper understanding of systemic issues, see:
- https://resolvemass.ca/common-bioanalytical-mistakes/
- https://resolvemass.ca/discovery-vs-regulated-bioanalysis/
- https://resolvemass.ca/why-is-bioanalysis-important/
1. Inadequate Audit Trails
Audit trail weaknesses are one of the most serious compliance findings in bioanalytical inspections.**
Common issues include:
- Disabled or partially configured system logs
- Lack of periodic audit trail review procedures
- Failure to investigate modified, deleted, or reintegrated chromatograms
- Shared user accounts preventing attribution
Without secure and reviewed audit trails, regulators may question whether reported results reflect the original data generated.
2. Spreadsheet-Based Calculations
Uncontrolled spreadsheets create significant vulnerability in Data Integrity in Bioanalytical studies.
Typical risks:
- Use of unvalidated Excel templates
- Manual transcription of instrument outputs
- Hidden formulas or overwritten cells
- No version control or change history
- Absence of independent verification
Even small formula errors can impact PK parameters, calibration curve calculations, and statistical outputs — potentially affecting regulatory decisions.
3. Sample Handling Errors
Chain-of-custody and storage controls are critical for defensible bioanalytical data.
Common documentation gaps:
- Missing sample receipt logs
- Incomplete chain-of-custody records
- Improper freezer temperature monitoring
- Unrecorded sample movement between storage locations
If sample integrity is questioned, the analytical data derived from those samples may also be challenged.
4. Selective Reporting
Selective data exclusion or undocumented reinjection practices severely compromise Data Integrity in Bioanalytical studies.
Regulators frequently identify:
- Reinjection of samples without scientific justification
- Omission of failed analytical runs from final reports
- Incomplete reporting of out-of-specification (OOS) results
- Reprocessing chromatograms without documentation
All generated data — including failures — must be retained and scientifically explained.
5. Insufficient Sponsor Oversight
Outsourcing does not transfer regulatory responsibility. Sponsors remain accountable for Data Integrity in Bioanalytical studies.
Oversight weaknesses include:
- No periodic quality audits of the CRO
- Lack of access to raw data or audit trails
- Overreliance on CRO quality assurances
- No defined governance structure for data review
Regulators expect sponsors to demonstrate active oversight, not passive delegation.
Sponsors outsourcing to:
- https://resolvemass.ca/bioanalytical-outsourcing/
- https://resolvemass.ca/managing-bioanalytical-cro-projects/
- https://resolvemass.ca/outsourced-bioanalysis-for-drug-development/
- https://resolvemass.ca/virtual-bioanalytical-strategy/
must actively maintain control.
Why Early Risk Identification Matters
Most data integrity failures do not begin as intentional misconduct. They often stem from:
- Inadequate training
- Weak system configuration
- Poor documentation culture
- Pressure to meet timelines
However, once patterns develop, remediation becomes costly and may delay IND, NDA, or BLA submissions.
Proactive risk assessment, structured oversight, and validated electronic systems are essential to protecting both regulatory compliance and scientific credibility.
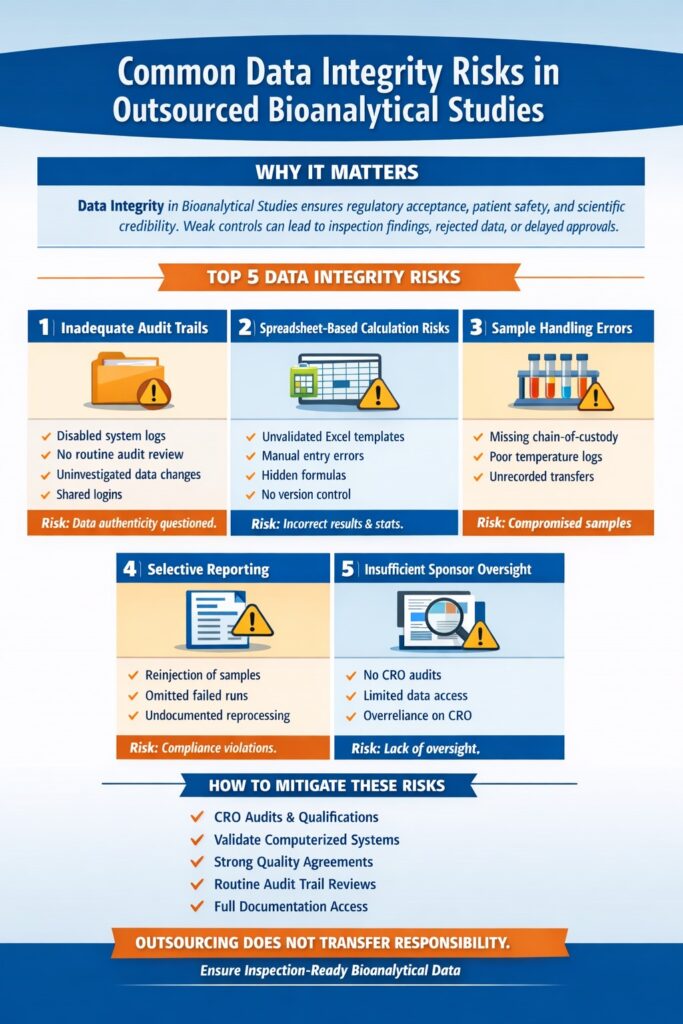
3: How Sponsors Can Ensure Data Integrity in Bioanalytical Studies
Sponsors must implement structured oversight, qualification processes, and quality agreements before and during outsourcing.
1. Perform Comprehensive CRO Qualification
Before outsourcing:
- Conduct on-site or remote audits
- Review previous regulatory inspection history
- Evaluate computerized system validation (CSV) documentation
- Verify training records
Key audit focus areas:
| Audit Area | What to Verify | Risk if Ignored |
|---|---|---|
| LIMS | Audit trail enabled and reviewed | Data manipulation risk |
| LC-MS systems | Secure user access | Unauthorized changes |
| SOPs | Version control & approval | Procedural deviations |
| Backup systems | Disaster recovery plan | Data loss |
Relevant technical capabilities:
- https://resolvemass.ca/lc-ms-ms-bioanalytical-services/
- https://resolvemass.ca/lc-ms-ms-bioanalysis-of-xenobiotics/
- https://resolvemass.ca/lc-ms-bioanalysis-for-oligonucleotides/
- https://resolvemass.ca/high-throughput-bioanalysis/
- https://resolvemass.ca/high-sensitivity-bioanalysis/
2. Establish Strong Quality Agreements
A quality agreement clearly defines responsibility for Data Integrity in Bioanalytical studies.
It should specify:
- Data ownership
- Record retention timelines
- Audit trail review procedures
- Deviation reporting timelines
- Sponsor access to raw data
Clear agreements prevent ambiguity during inspections.
3. Ensure Computerized System Validation (CSV)
Validated electronic systems are mandatory for maintaining Data Integrity in Bioanalytical studies.
Critical systems include:
- LIMS
- Chromatography data systems
- Electronic lab notebooks
- Statistical software
Sponsors should verify:
- Installation Qualification (IQ)
- Operational Qualification (OQ)
- Performance Qualification (PQ)
- Change control procedures
Related regulated services:
- https://resolvemass.ca/regulated-bioanalytical-services/
- https://resolvemass.ca/clinical-bioanalytical-services/
- https://resolvemass.ca/toxicokinetic-bioanalysis/
- https://resolvemass.ca/pk-pd-bioanalysis/
- https://resolvemass.ca/pk-tk-bioanalysis/
4. Implement Routine Sponsor Oversight
Oversight should be proactive, not reactive.
Best practices:
- Quarterly quality reviews
- Random raw data checks
- Audit trail review sampling
- Ongoing communication meetings
Startups and growing biotech companies can explore:
- https://resolvemass.ca/affordable-bioanalytical-services-for-biotech-startups/
- https://resolvemass.ca/outsource-bioanalysis-for-biotech-startups/
- https://resolvemass.ca/cost-effective-bioanalytical-services/
- https://resolvemass.ca/bioanalytical-testing-services-cost/
A collaborative partnership reduces risk significantly.
4: Role of Documentation in Data Integrity in Bioanalytical Studies
Documentation is evaluated as rigorously as analytical performance in Data Integrity in Bioanalytical studies. Even scientifically accurate results can be rejected if documentation is incomplete, inconsistent, or not inspection-ready.
Regulatory agencies such as the U.S. Food and Drug Administration and Health Canada assess whether every reported value is fully traceable to original raw data and supported by controlled records.
Why Documentation Is Central to Data Integrity
Proper documentation ensures:
- Full traceability from sample receipt to final report
- Transparent justification for deviations or reanalysis
- Reconstruction of study activities during inspections
- Demonstration of ALCOA+ compliance
If an inspector cannot reconstruct the analytical workflow from documentation alone, the integrity of the study may be questioned.
Critical Documentation in Bioanalytical Studies
The following records are essential to maintaining Data Integrity in Bioanalytical studies:
1. Method Validation Reports
- Accuracy, precision, selectivity, stability data
- Calibration model justification
- Matrix effect assessments
- Acceptance criteria and statistical analysis
These reports prove the method is scientifically fit for purpose.
2. Analytical Run Summaries
- Run sequence documentation
- Sample placement and reinjection records
- System suitability results
- QC performance evaluation
All accepted and rejected runs must be documented with scientific justification.
3. Calibration Curves
- Raw instrument output
- Back-calculated concentrations
- Regression model selection
- Acceptance/rejection rationale
Improperly documented calibration decisions are common inspection findings.
4. QC Acceptance Criteria
- Predefined acceptance ranges
- Justification for any deviation
- Documentation of repeat analysis
Changing acceptance criteria retrospectively is a major compliance violation.
5. Deviation Investigations
- Root cause analysis
- Impact assessment on study data
- Corrective and preventive actions (CAPA)
- QA approval and closure documentation
Incomplete investigations create regulatory concern regarding systemic weaknesses.
Advanced and complex modalities require strong documentation systems, especially for:
- https://resolvemass.ca/advanced-bioanalytical-strategies-for-complex-drug-modalities/
- https://resolvemass.ca/antibody-drug-conjugate-bioanalytical-services/
- https://resolvemass.ca/cell-and-gene-therapy-bioanalysis/
- https://resolvemass.ca/biosimilar-bioanalysis/
- https://resolvemass.ca/proteomics-bioanalytical-services/
- https://resolvemass.ca/biomarker-bioanalytical-services/
- https://resolvemass.ca/biomarker-bioanalytical-services-2/
- https://resolvemass.ca/bioanalytical-stability-testing/
Technology integration also plays a role:
Incomplete documentation can invalidate otherwise accurate data and delay submissions.
Regulatory Perspective
During inspections, regulators evaluate whether:
- Records are contemporaneous
- Corrections are properly justified and traceable
- Electronic and paper records align
- Audit trails support reported results
Incomplete documentation can invalidate otherwise accurate data, leading to study rejection, reanalysis requests, or regulatory delays.
Key Takeaway
In Data Integrity in Bioanalytical studies, documentation is not administrative paperwork — it is scientific evidence. Robust, transparent, and inspection-ready documentation safeguards regulatory approval and protects the credibility of outsourced bioanalytical programs.
5: Case-Based Perspective: Lessons from Regulatory Actions
Regulatory enforcement actions globally demonstrate a common theme:
- Data manipulation often starts small
- Weak oversight allows patterns to develop
- Documentation gaps raise suspicion
Organizations that embed compliance from the beginning avoid costly remediation.
ResolveMass Laboratories Inc. emphasizes:
- Transparent audit trail review
- Strong SOP governance
- Cross-functional quality oversight
- Client-accessible documentation
Full overview of capabilities:
👉 https://resolvemass.ca/resolvemass-bioanalytical-services-overview/
👉 https://resolvemass.ca/bioanalytical-laboratory-services/
👉 https://resolvemass.ca/bioanalytical-cro-for-drug-discovery/
👉 https://resolvemass.ca/bioanalytical-cro-services-for-pk-and-tk/
This structured framework supports reliable and inspection-ready data packages.
6: How ResolveMass Laboratories Inc. Ensures Data Integrity in Bioanalytical Studies
ResolveMass integrates scientific rigor with compliance-driven systems to protect data integrity at every stage.
Key strengths include:
- Experienced bioanalytical scientists
- Fully validated LC-MS/MS platforms
- Controlled electronic data systems
- Independent QA review processes
- Sponsor-accessible raw data
Our team understands regulatory expectations across North America and Europe, ensuring your outsourced program withstands scrutiny.
Conclusion:
Data Integrity in Bioanalytical studies is not optional — it is a regulatory, ethical, and scientific requirement.
In outsourced environments, sponsors must go beyond contractual agreements and actively verify systems, documentation, and cultural commitment to compliance.
By implementing:
- Strong CRO qualification
- Validated computerized systems
- Clear quality agreements
- Ongoing oversight
You significantly reduce regulatory risk and protect your development timelines.
ResolveMass Laboratories Inc. stands as a trusted partner committed to ensuring uncompromised Data Integrity in Bioanalytical studies from sample receipt to final report submission.
Frequently Asked Questions:
Data Integrity in Bioanalytical studies refers to the accuracy, completeness, consistency, and reliability of data throughout its lifecycle. It ensures that analytical results are attributable, legible, contemporaneous, original, and accurate (ALCOA+ principles). Regulatory agencies require full traceability from raw data to reported results. Any manipulation, deletion, or undocumented changes can lead to serious compliance issues. Strong data integrity ensures regulatory acceptance and patient safety.
Regulators such as the U.S. Food and Drug Administration and European Medicines Agency evaluate both scientific accuracy and documentation integrity. Even technically valid results can be rejected if audit trails or documentation are incomplete. Data integrity failures can lead to warning letters, study rejection, or delays in approval. Agencies expect full transparency and traceability. Maintaining integrity protects both sponsors and study credibility.
Common risks include disabled audit trails, shared user logins, inadequate access controls, and poor documentation practices. Lack of computerized system validation (CSV) can also compromise data reliability. Incomplete deviation reporting and weak SOP controls are additional risk factors. Failure to back up data properly may result in permanent loss. These gaps can trigger regulatory findings during inspections.
Sponsors should perform comprehensive CRO qualification audits before outsourcing. Reviewing inspection history, training records, and system validation documents is essential. Strong quality agreements must clearly define responsibilities and audit trail review processes. Routine oversight such as raw data checks and quarterly quality meetings strengthens compliance. Proactive collaboration reduces regulatory risk significantly.
Critical systems include LIMS, chromatography data systems, electronic lab notebooks, and statistical software. Each system must undergo Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). Change control procedures must be documented and controlled. Access permissions should be role-based and secure. Proper validation ensures data remains accurate and tamper-proof.
If violations are found, regulators may issue warning letters or request data exclusion. In severe cases, studies may be rejected or sponsors may need to repeat studies. This can lead to financial losses and significant development delays. Reputational damage is also a major concern. Early detection and corrective action are critical to minimize impact.
Reference
- Tianke Wang & Arkady I Gusev. Ensuring Quality and Compliance in Outsourcing of Bioanalysis of Clinical Biomarkers.https://www.tandfonline.com/doi/full/10.4155/bio-2017-0008
- Christopher Tudan. Good Clinical Practices in the Bioanalytical Laboratory.https://www.tandfonline.com/doi/abs/10.4155/bio-2023-0150
- Yue-Ting Wang ,Estelle M Maes, Lance Heinle, Kenneth Ruterbories, Stella Doktor, Mary Larsen. Integrity and Efficiency: AbbVie’s Journey of Building an Integrated Nonregulated Bioanalytical Laboratory.https://www.tandfonline.com/doi/abs/10.4155/bio-2023-0012
- Tom Verhaeghe. Bioanalytical Outsourcing Strategy at Janssen Research and Development.https://www.tandfonline.com/doi/abs/10.4155/bio.14.105
- Melanie Anderson. Ensuring Biological Sample Integrity from Collection to Analysis for LC–MS Workflows: Case Studies Illustrating Challenges in Clinical Trials.https://www.tandfonline.com/doi/abs/10.4155/bio-2019-0176

