Introduction
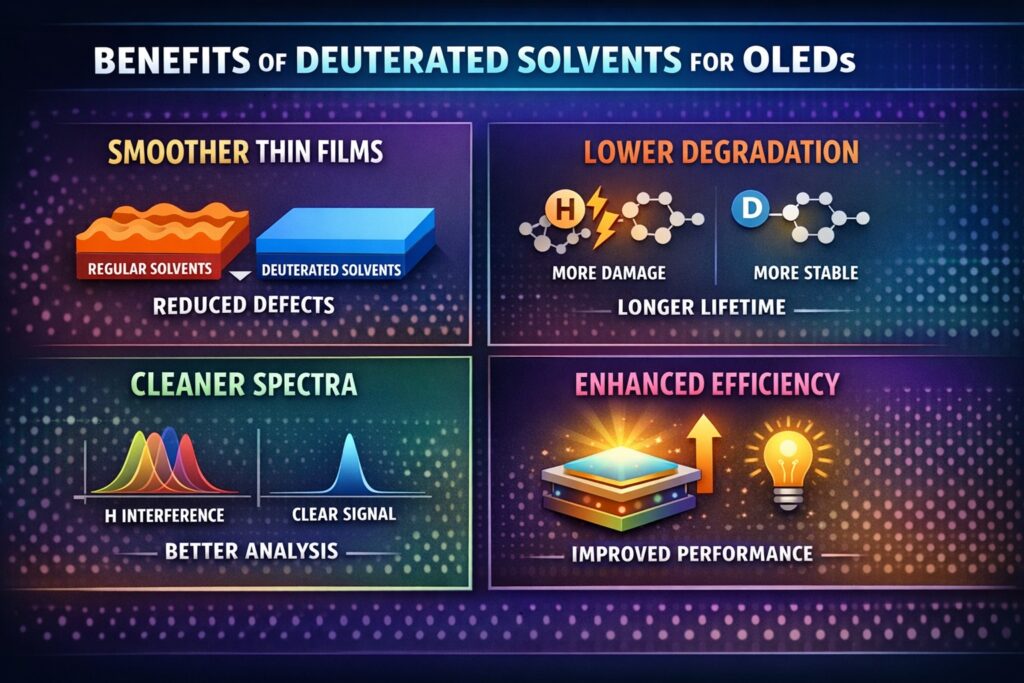
Deuterated Solvents for OLED fabrication are increasingly used to improve film quality, limit hydrogen-related degradation, and enhance both device lifetime and analytical accuracy. In modern OLED manufacturing, especially in solution-based and research-driven processes, even small chemical differences can strongly influence charge transport, exciton behavior, and operational stability. Using deuterated solvents and aromatics introduces a higher level of chemical control during fabrication.
In multilayer OLED stacks, device performance depends not only on emitter molecules but also on the chemical environment during deposition. Deuterated solvents slightly modify vibrational energy transfer, evaporation behavior, and intermolecular interaction while the film forms. These subtle changes improve thin-film uniformity and long-term reliability. As OLED technology advances, precise control over processing chemistry becomes more important for consistent results.
This article explains how deuterated solvents and aromatics function in practical OLED fabrication. The focus is on real device engineering benefits and measurable performance improvements rather than theoretical discussion.
Learn more about the broader impact of isotopic labeling: Explore Stable Isotope Labeled Compounds
How Do Deuterated Solvents for OLED Influence Thin-Film Morphology?
Deuterated Solvents for OLED processing influence thin-film morphology by adjusting solvent–solute interaction, drying speed, and molecular packing during deposition. While these changes occur at the molecular level, they directly affect film smoothness and device efficiency. Stable morphology is essential for consistent electrical and optical performance.
In solution-processed methods such as spin-coating, slot-die coating, and inkjet printing, film structure determines charge balance and exciton confinement. Small differences in solvent behavior can cause phase separation, crystallization, or defect formation. Over time, these issues reduce brightness stability and device lifetime. By using isotopically substituted solvents, engineers can achieve more controlled drying and improved molecular organization.
Access high-quality materials for your fabrication process: Buy Deuterated Compounds for Advanced Research
Key Technical Mechanisms
- Modified Hydrogen Bonding Strength
- C–D bonds have lower vibrational energy than C–H bonds.
- This slightly changes intermolecular interaction during solvent evaporation.
- It allows better control of microphase separation in host–guest systems.
- The result is smoother films and improved material compatibility.
- Reduced Proton Exchange Contamination
- Protic impurities can accelerate chemical degradation.
- Deuterated solvents reduce unwanted hydrogen exchange reactions.
- This lowers defect formation that can act as charge traps.
- Cleaner films help maintain stable electrical properties.
- Controlled Evaporation Behavior
- Isotopic substitution can slightly affect vapor pressure.
- Slower and more uniform evaporation supports better self-assembly.
- Improved drying reduces thickness variation.
- This leads to better reproducibility across devices.
Impact on Device Parameters
| Fabrication Factor | Influence of Deuterated Solvents | Device Impact |
|---|---|---|
| Drying kinetics | More uniform solvent evaporation | Reduced pinholes |
| Host–guest mixing | Improved miscibility stability | Higher EQE |
| Molecular orientation | Better horizontal alignment | Increased light outcoupling |
| Trap-state density | Lower hydrogen-induced defects | Reduced leakage current |
Ensure the highest precision in your chemical standards: View our Deuterated Internal Standards
Why Are Deuterated Aromatics Critical in OLED Emitter Systems?
Deuterated aromatics improve exciton stability and reduce energy loss in phosphorescent and TADF OLED systems. Their main benefit comes from lowering vibrational quenching, which is a key reason for efficiency loss. In high-performance devices, reducing non-radiative decay is essential for maintaining strong light emission.
Inside OLED materials, non-radiative decay often occurs through vibrational coupling involving C–H bonds. When hydrogen is replaced by deuterium in aromatic structures, vibrational frequency decreases. This reduces energy loss pathways and improves exciton confinement. The improvement is especially important for blue emitters, which typically face greater stability challenges.
Discover why Benzene-D6 is a game-changer for OLEDs: Why Benzene-D6 Improves Stability in OLED
Suppression of Non-Radiative Decay
Because C–D bonds vibrate more slowly than C–H bonds:
- Vibrational energy loss decreases.
- Triplet exciton stability improves.
- Internal quantum efficiency increases.
- Thermal degradation slows down.
These effects are valuable in blue phosphorescent, TADF, and hyperfluorescent OLED architectures where efficiency and stability must be balanced carefully.
Find reliable aromatic precursors for your display technology: Deuterated Aromatic Compounds for OLED
Improved Chemical Stability
Hydrogen abstraction is a known degradation pathway in OLED materials. Deuterated aromatics:
- Increase effective bond strength.
- Slow radical-based degradation.
- Improve resistance to exciton-induced bond breaking.
- Extend operational lifetime (LT50).
These factors help maintain brightness and reduce performance drop during long operation.
Role of Deuterated Solvents for OLED in Spectroscopic and Process Control
Deuterated Solvents for OLED fabrication support accurate analytical testing and reproducible process control. During material synthesis and device optimization, precise characterization is essential. Even very small impurities can change electrical behavior and lifetime results.
Advanced OLED development depends on tools such as NMR, FTIR, and photoluminescence spectroscopy. When deuterated solvents are used, hydrogen signal interference is minimized. This creates cleaner spectral baselines and allows better impurity detection. As a result, batch-to-batch consistency improves and process validation becomes more reliable.
For research laboratories and pilot production lines, this level of analytical clarity is critical. It ensures that performance differences are due to material design rather than contamination or measurement error.
Optimize your analytical workflows with specialized solvents: Deuterated Standards and Solvents for NMR
How Do Deuterated Solvents for OLED Affect Device Lifetime?
Deuterated Solvents for OLED can indirectly improve operational lifetime by reducing hydrogen-related degradation and supporting uniform film structure. Device stability depends strongly on chemical robustness and defect control. Lower hydrogen activity helps slow chemical breakdown inside emissive layers.
Common OLED degradation pathways include exciton-induced bond cleavage, radical formation, hydrogen abstraction, and interfacial oxidation. When deuterated materials are incorporated, bond breaking rates decline and radical propagation slows. This delays material degradation and improves long-term performance.
Observed Improvements
Studies on isotopic substitution have reported:
- Longer LT50 lifetime values.
- Reduced dark spot formation.
- Slower increase in leakage current.
- Better brightness stability over time.
These improvements are highly relevant for high-brightness displays, automotive panels, and micro-OLED applications.
Partner with a leading specialist for your material needs: Deuterated Labelled Chemical Synthesis Company in United States
Deuterated Solvents for OLED in Solution-Processed Architectures
Deuterated Solvents for OLED are particularly useful in inkjet and roll-to-roll processing, where solvent behavior directly defines film quality. In these methods, solvent properties control layer thickness, interfacial integrity, and pattern precision. Small variations can strongly influence final device performance.
Key solvent parameters include surface tension, boiling point, and solubility compatibility. Deuterated solvents can provide improved wetting on PEDOT:PSS layers and reduce coffee-ring effects during printing. They also help maintain more uniform thickness and predictable drying patterns.
In multilayer fabrication, solvent orthogonality is essential to prevent damage to underlying layers. Carefully selected deuterated aromatics can support stable interfaces and protect electrical performance.
Integration of Deuterated Aromatics in Host Materials
Deuterated aromatic host materials enhance exciton stability and reduce vibrational energy loss in high-efficiency OLED stacks. Host matrices influence charge balance, triplet energy alignment, and exciton confinement. Their chemical stability directly affects overall device durability.
Replacing hydrogen with deuterium in host materials reduces vibrational coupling losses and stabilizes high-energy blue emitters. It also minimizes exciton-polaron quenching and supports efficiency retention during long operation. These improvements are particularly important in advanced TADF and blue OLED systems.
Manufacturing-Scale Considerations for Deuterated Solvents for OLED
At production scale, Deuterated Solvents for OLED must meet strict purity standards to avoid yield loss and device failure. Even trace moisture or metal contamination can reduce consistency across large batches. High purity ensures reliable luminance output and stable electrical behavior.
Critical quality parameters include:
- ≥99.5% isotopic purity
- Very low moisture levels (ppm range)
- Strict trace metal limits
- Verified GC-MS impurity profiles
Maintaining these standards supports reproducibility in both R&D and pilot manufacturing environments. Secure sourcing and analytical certification are essential for long-term reliability.
Secure a consistent supply for your production line: Trusted Supplier of Deuterated Reagents
Best Practices for Using Deuterated Solvents for OLED Fabrication
- Store materials under inert atmosphere to prevent moisture exposure.
- Avoid hydrogen contamination during transfer and handling.
- Verify isotopic purity before sensitive experiments.
- Maintain controlled humidity in fabrication areas.
- Perform regular GC-MS and NMR quality checks.
- Keep batch records for full traceability and quality assurance.
Future Outlook: Isotopic Engineering in OLEDs
As OLED structures become thinner and more complex, isotopic engineering may play a larger role in performance optimization. Controlling vibrational energy at the molecular level offers a new pathway to enhance efficiency and stability.
Future developments may include fully deuterated emissive stacks, engineered host–guest systems, and advanced blue emitter stabilization. With ongoing research, isotopic design could become a standard strategy in next-generation OLED manufacturing.
Conclusion
Deuterated Solvents for OLED fabrication provide measurable benefits in film control, reduced non-radiative decay, improved analytical precision, and longer device lifetime. Through careful isotopic substitution, both solvents and aromatic materials enhance stability, efficiency, and reproducibility.
For advanced OLED systems such as blue emitters, TADF devices, and solution-processed architectures, deuterated materials create a cleaner and more controlled chemical environment. As device requirements continue to tighten, isotopic precision will become increasingly important in high-performance OLED engineering.
For technical inquiries or material specifications, you can connect with our team:
Contact Us
Frequently Asked Questions (FAQs)
Deuterated solvents slightly change how molecules interact and how the solvent evaporates during coating. This helps the film dry in a more uniform way, reducing defects such as pinholes and phase separation. As a result, the emissive layer becomes smoother and more stable. Better film quality directly supports improved efficiency and device consistency.
Yes, deuterated aromatics can support longer device lifetime by lowering vibrational energy losses inside the material. This reduces stress on excited states and slows down chemical breakdown. Over time, this helps maintain brightness and color stability. The overall effect is improved operational durability.
They are not mandatory for every OLED product, but they are highly valuable in advanced or high-performance applications. Research labs and manufacturers working on blue or long-lifetime devices often benefit the most. Their use depends on performance goals and cost considerations. In many R&D settings, they provide a clear technical advantage.
They can indirectly support higher external quantum efficiency by improving film morphology and reducing defect-related quenching. When the emissive layer is more uniform, charge balance becomes more stable. This allows a higher percentage of excitons to produce light. The improvement depends on overall device design.
Yes, they can influence charge transport by reducing impurity-related trap states in the film. Fewer defects allow charges to move more smoothly across layers. This supports better charge balance and reduces leakage current. Overall electrical stability may improve as a result.
Yes, they are well suited for inkjet and other solution-based printing techniques. Their controlled evaporation behavior can help improve droplet formation and drying patterns. This reduces common issues such as coffee-ring effects. More uniform printed layers lead to better device performance.
Reference
- Munir, R., Zahoor, A. F., Khan, S. G., Hussain, S. M., Noreen, R., Mansha, A., Hafeez, F., Irfan, A., & Ahmad, M. (2025, August 21). Total syntheses of deuterated drugs: A comprehensive review. Top Current Chemistry (Cham), 383(3), 31. https://doi.org/10.1007/s41061-025-00515-x
- Di Martino, R. M., Maxwell, B. D., & Pirali, T. (2023). Deuterium in drug discovery: Progress, opportunities and challenges. Nature Reviews Drug Discovery, 22(7), 562–584. https://doi.org/10.1038/s41573-023-00703-8
- Kopf, S., Bourriquen, F., Li, W., … & Morandi, B. (2022). Recent developments for the deuterium and tritium labeling of organic molecules. Chemical Reviews, 122(6), 6634-6713. https://doi.org/10.1021/acs.chemrev.1c00795


