Summary
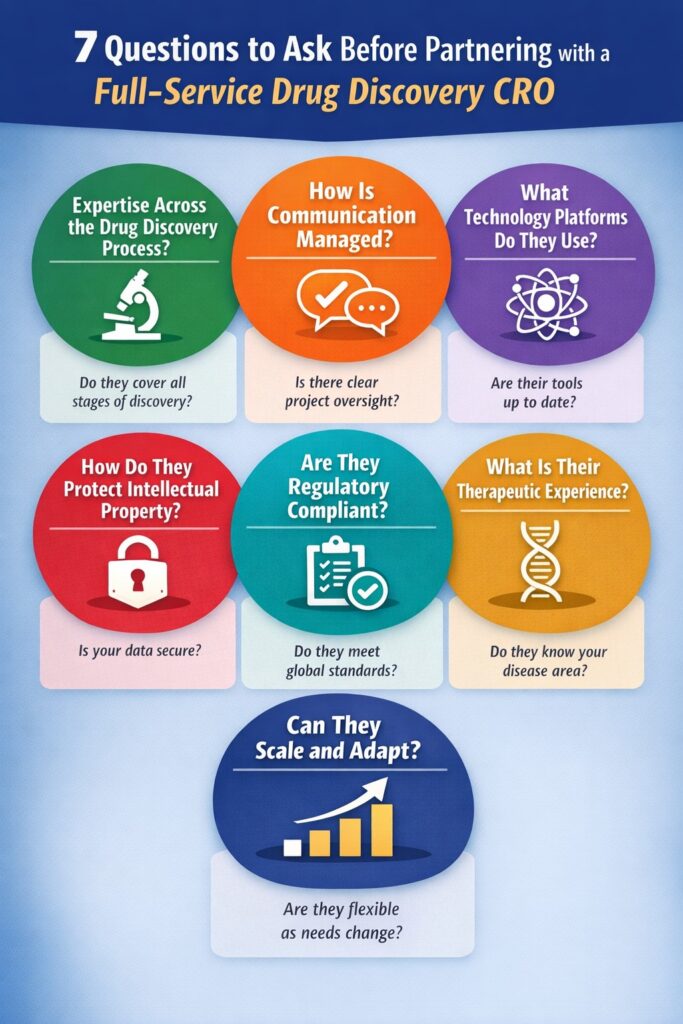
Before partnering with a Full Service Drug Discovery CRO, the following key questions can help you evaluate technical depth, transparency, compliance, and innovation:
- How comprehensive is their scientific and operational expertise?
- Do they maintain transparent communication and project management?
- What technologies and discovery platforms support their research?
- How do they ensure IP protection and data integrity?
- Are they compliant with global regulatory standards (GLP, GCP, GMP)?
- What is their track record in therapeutic area success?
- How adaptable are they to evolving project needs and timelines?
Each of these questions reveals whether your potential Full Service Drug Discovery CRO can deliver reliable, efficient, and compliant solutions that advance your molecule from concept to clinic.
Introduction: Choosing the Right Full Service Drug Discovery CRO
Selecting the right Full Service Drug Discovery CRO is one of the most important decisions in preclinical development. This decision directly affects development timelines, scientific quality, regulatory acceptance, and overall return on investment. Choosing the wrong CRO can lead to data gaps, repeated studies, delays, and increased downstream risk.
Although many CROs promote “end-to-end” drug discovery services, true value lies in real scientific depth, operational discipline, and alignment with sponsor goals. Sponsors should look beyond marketing messages and focus on proven performance, transparency, and data reproducibility. These factors often determine whether a program moves forward smoothly or faces major setbacks.
In this article, we outline seven essential questions every sponsor should ask before engaging a Full Service Drug Discovery CRO. These questions are designed to support confident decision-making while reducing scientific, regulatory, and operational risks throughout the discovery pipeline.
Accelerate your research with a partner that understands molecular complexity. Explore our Custom Synthesis for Drug Discovery to fuel your pipeline.
1. How Deep Is Their Expertise Across the Drug Discovery Continuum?
Answer upfront: A reliable Full Service Drug Discovery CRO should show proven expertise across hit identification, lead optimization, ADME, toxicology, and preclinical validation. This depth supports continuity and reduces errors between discovery stages.
Sponsors should look for CROs that demonstrate successful program progression, not just isolated services. Experience across different target classes, modalities, and disease biology is especially valuable when dealing with complex research challenges. Strong scientific leadership also promotes hypothesis-driven decisions instead of trial-and-error approaches.
When evaluating technical depth, consider the following:
- Request detailed case studies showing smooth transitions between discovery phases
- Review in-house assay capabilities, including biochemical, cell-based, and in vivo models
- Examine scientist qualifications, publications, and conference participation
- Confirm therapeutic area expertise such as oncology, CNS, or immunology
Tip: A Full Service Drug Discovery CRO with integrated biology, chemistry, and DMPK teams minimizes data silos and improves confidence in decision-making across the program lifecycle.
Unlock precise molecular insights with industry-leading technology. Discover how our High Resolution Mass Spectrometry (HRMS) Analysis can elevate your project.
2. What Is Their Communication and Project Management Structure?
Answer upfront: A dependable Full Service Drug Discovery CRO prioritizes clear communication, structured reporting, and defined milestones. Strong project management keeps sponsors aligned with ongoing experimental work.
Transparent communication helps avoid delays, prevents scope creep, and supports fast problem-solving. CROs with mature governance systems allow sponsors to make timely decisions at key development points. In contrast, poor communication often leads to rework and misaligned expectations.
Signs of effective project management include:
- A dedicated Project Manager or single point of contact
- Weekly or bi-weekly progress reports with clear action items
- Shared digital dashboards for real-time data tracking
- Defined escalation paths and risk mitigation plans
A well-structured communication system strengthens collaboration and keeps discovery programs focused and efficient.
Best practice: Ask to review Project Management SOPs to understand reporting standards and workflow transparency.
3. Which Technology Platforms and Discovery Tools Do They Use in a Full Service Drug Discovery CRO?
Answer upfront: Technology directly affects discovery speed, data quality, and downstream success. A modern Full Service Drug Discovery CRO invests in advanced platforms to improve predictability and reduce late-stage failures.
Today’s discovery programs rely on automation, AI tools, and integrated data systems. A CRO’s technology investments reflect its commitment to quality and innovation. Outdated tools can limit scalability and compromise reproducibility.
Technology Capabilities to Evaluate
| Technology Domain | Key Indicators of Excellence |
|---|---|
| High-Throughput Screening (HTS) | Automation-enabled workflows, AI-based hit selection |
| Computational Chemistry / AI Modeling | Predictive docking, molecular dynamics, ADMET modeling |
| Proteomics & Genomics Integration | Multi-omics data for stronger target validation |
| In Vivo Translational Models | Disease-relevant and humanized efficacy models |
Leading Full Service Drug Discovery CROs combine AI-driven platforms with cloud-based informatics to improve traceability, reproducibility, and decision speed.
Pro tip: CROs with strong digital lab infrastructure often deliver better data and faster timelines.
Ensure your data meets the highest clinical and regulatory standards. Learn more about our comprehensive Bioanalytical Services.
4. How Do They Protect Intellectual Property and Data Integrity?
Answer upfront: A trustworthy Full Service Drug Discovery CRO must follow strict IP protection policies and maintain robust data integrity systems. These safeguards are essential for regulatory submissions and future partnerships.
Sponsors should confirm that all data remains confidential, secure, and fully traceable. Weak data governance increases audit risk and can threaten competitive advantage. Secure systems also support long-term data usability.
Key areas to review include:
- Clear confidentiality and non-disclosure agreements defining data ownership
- Recognized data security standards such as ISO 27001
- Electronic Lab Notebooks (ELNs) with audit trails and version control
- Role-based access controls for sensitive information
Sponsors should always retain exclusive IP rights for all data and compounds generated.
Warning: CROs lacking documented data governance systems pose serious risks during audits or regulatory reviews.
Identify and quantify contaminants before they impact your timeline. Review our expert Impurity Profiling using LCMS services today.
5. Is the Full Service Drug Discovery CRO Compliant with Global Regulatory Standards?
Answer upfront: Regulatory compliance is the foundation of credible discovery data. A compliant Full Service Drug Discovery CRO ensures that generated data meets global regulatory expectations and supports IND-enabling activities.
Non-compliant data may lead to study rejection, repeated experiments, and costly delays. Sponsors should confirm that compliance is built into daily operations, not just stated in marketing materials.
Regulatory compliance checklist:
- GLP-certified facilities for toxicology and safety studies
- AAALAC accreditation for animal welfare
- GMP partnerships for clinical material production
- Openness to sponsor and regulatory audits
Always request compliance certificates and recent audit reports.
Note: Regulatory gaps can result in data rejection by agencies such as the FDA or EMA.
6. What Is Their Track Record in Relevant Therapeutic Areas?
Answer upfront: Therapeutic area experience strongly influences a Full Service Drug Discovery CRO’s ability to interpret complex biology and generate meaningful data.
CROs with disease-specific expertise can anticipate challenges, design better assays, and apply deeper mechanistic understanding. This experience often leads to faster timelines and improved translational outcomes.
Important questions to ask:
- Have they advanced compounds in your target indication?
- Do they publish or present at disease-focused conferences?
- Can they show examples where expertise reduced development time?
Top-performing Full Service Drug Discovery CROs often maintain proprietary disease models and specialized assays.
Pro insight: Case studies reveal real-world scientific judgment and data reproducibility.
Trace your molecule’s path with unparalleled precision. Find out how our Isotopic Labeling Services support advanced drug discovery.
7. How Flexible and Scalable Is the Full Service Drug Discovery CRO?
Answer upfront: An effective Full Service Drug Discovery CRO must adapt quickly as project needs change and scale resources without sacrificing quality.
Discovery programs evolve based on data. A flexible CRO maintains momentum, while a rigid one becomes a bottleneck. Scalability is especially important for accelerated programs or expanding pipelines.
Indicators of flexibility include:
- Ability to rapidly scale teams and infrastructure
- Modular service offerings with milestone-based contracts
- Cross-functional teams that maintain quality under pressure
Operational agility ensures progress even during unexpected scientific or logistical challenges.
Recommendation: Choose CROs that offer hybrid collaboration models for maximum flexibility.
Bonus: Decision Framework for Selecting a Full Service Drug Discovery CRO
| Evaluation Parameter | Ideal CRO Traits | Questions to Ask |
|---|---|---|
| Scientific Expertise | Multidisciplinary teams with long-term experience | How do biology and chemistry teams collaborate? |
| Communication | Clear, milestone-based reporting | How often are project reviews held? |
| Technology | AI-enabled screening and bioinformatics | How do you ensure data reproducibility? |
| Compliance | Certified GLP/GCP facilities | Can you share recent audit reports? |
| Flexibility | Custom engagement models | How do you manage project changes? |
This framework supports balanced evaluation before finalizing a CRO partnership.
Conclusion: Partnering with the Right Full Service Drug Discovery CRO
Before collaborating with any Full Service Drug Discovery CRO, these seven questions should guide your evaluation process. The right CRO acts as a strategic R&D partner, not just a service provider.
Scientific expertise, modern infrastructure, regulatory readiness, and operational flexibility together define partnership success. Selecting a CRO with proven excellence and transparency greatly improves the chances of moving from discovery to the clinic efficiently.
Ready to streamline your path to the clinic? Connect with our team to discuss your tailored drug discovery strategy.
For consultation or partnership discussions, reach out through:
👉 Contact Us
FAQs About Collaborating with a Full Service Drug Discovery CRO
A Full Service Drug Discovery CRO provides end-to-end discovery support, starting from target validation and hit identification through lead optimization and IND-enabling studies. This integrated approach ensures scientific continuity and consistent data quality across all stages. It also reduces the need to manage multiple vendors.
Integrated CROs streamline workflows by keeping biology, chemistry, DMPK, and toxicology under one roof. This reduces communication gaps and shortens development timelines. Sponsors benefit from faster decision-making and better data alignment across discovery phases.
Credibility can be assessed by reviewing regulatory certifications, audit history, and past project outcomes. Client testimonials, case studies, and published scientific work also provide valuable insight. Transparent documentation is a strong indicator of reliability.
A well-structured contract should define IP ownership, data access rights, confidentiality terms, and milestone-based deliverables. It should also outline timelines, reporting standards, and termination clauses. Clear contracts help prevent misunderstandings during long-term collaborations.
Common risks include limited visibility into experimental work, IP exposure, and inconsistent data quality. These risks can be reduced by selecting a CRO with strong governance systems. Regular communication and clear data ownership policies are essential.
Reference
- Steadman, V. A. (2018). Drug discovery: Collaborations between contract research organizations and the pharmaceutical industry. ACS Medicinal Chemistry Letters, 9(7), 581–583. https://doi.org/10.1021/acsmedchemlett.8b00236
- ScienceDirect Topics. (n.d.). Contract research organization. Elsevier. Retrieved January 12, 2026, from https://www.sciencedirect.com/topics/pharmacology-toxicology-and-pharmaceutical-science/contract-research-organization


