Introduction
GC-MS analysis for pharmaceuticals is the gold standard technique for identifying and quantifying volatile and semi-volatile compounds in drug substances and products. Gas chromatography-mass spectrometry combines the separation power of gas chromatography with the identification capability of mass spectrometry, making it indispensable for pharmaceutical quality control, research, and development.
To understand the fundamentals, read our detailed overview on Gas Chromatography–Mass Spectrometry:
https://resolvemass.ca/gas-chromatography-mass-spectrometry/
and the Working Principle of GC-MS:
https://resolvemass.ca/working-principle-of-gc-ms/
At ResolveMass Laboratories Inc., we understand that pharmaceutical companies face increasing pressure to ensure product safety, efficacy, and regulatory compliance. Our custom GC-MS analysis for pharmaceuticals delivers precise, reliable data that supports critical decisions throughout the drug development lifecycle. From early-stage research to commercial manufacturing, our analytical expertise helps pharmaceutical clients meet the most stringent quality standards.
We provide GC-MS analysis services across North America, including specialized support in the United States:
https://resolvemass.ca/gcms-analysis-in-united-states/
and Montreal, Canada:
https://resolvemass.ca/gcms-analysis-in-montreal/
The pharmaceutical industry relies on GC-MS analysis for pharmaceuticals to detect impurities at trace levels, understand degradation pathways, and validate product stability. This article explores how custom GC-MS methodologies address three essential aspects of pharmaceutical analysis: impurity profiling, forced degradation studies, and stability testing.
A broader overview of GC-MS applications can be found here:
https://resolvemass.ca/applications-of-gcms/
Summary
Custom GC-MS analysis for pharmaceuticals provides critical analytical data for drug safety, quality control, and regulatory compliance. This comprehensive guide covers:
- Impurity Profiling: GC-MS analysis identifies and quantifies process-related impurities, degradation products, and contaminants in pharmaceutical compounds with detection limits as low as 0.01%
- Forced Degradation Studies: Systematic stress testing under thermal, oxidative, photolytic, and hydrolytic conditions reveals potential degradation pathways and supports stability-indicating method development
- Stability Studies: Long-term and accelerated stability testing using GC-MS analysis ensures pharmaceutical products maintain quality, safety, and efficacy throughout their shelf life
- Regulatory Compliance: Custom GC-MS methods meet ICH, FDA, USP, and EP guidelines for pharmaceutical analysis and documentation
- Method Development: Tailored analytical approaches address unique challenges in small molecules, biologics, and complex formulations
Residual solvent testing is a major component of impurity profiling and is discussed in detail in our article on GC-MS Residual Solvent Analysis:
https://resolvemass.ca/gcms-residual-solvent-analysis-what-you-must-know/
Our complete analytical offerings are available under GC-MS Analysis Services:
https://resolvemass.ca/gcms-analysis-service/
1: Understanding GC-MS Technology in Pharmaceutical Applications
GC-MS combines gas chromatography separation with mass spectrometry detection to provide both qualitative and quantitative analysis of pharmaceutical compounds. This dual capability makes it superior to standalone techniques for complex pharmaceutical matrices.
Core Advantages of GC-MS Analysis for Pharmaceuticals
- High Sensitivity: Detection limits down to parts-per-billion (ppb) levels
- Selectivity: Mass spectrometry provides structural information for unknown compounds
- Versatility: Applicable to small molecules, excipients, solvents, and volatile degradation products
- Regulatory Acceptance: Widely recognized and accepted by FDA, EMA, and ICH guidelines
- Quantitative Precision: Excellent linearity and reproducibility for trace analysis
A step-by-step technical explanation is available in our guide on the Working Principle of GC-MS:
https://resolvemass.ca/working-principle-of-gc-ms/
GC-MS Instrumentation for Pharmaceutical Analysis
| Component | Function | Pharmaceutical Application |
|---|---|---|
| Gas Chromatograph | Separates compounds based on volatility and polarity | Resolves complex pharmaceutical mixtures |
| Mass Spectrometer | Identifies compounds by mass-to-charge ratio | Structural elucidation of impurities |
| Detector Types | Quadrupole, Ion Trap, TOF, Triple Quad | Selected based on sensitivity and specificity needs |
| Data System | Processes spectral data and generates reports | Ensures regulatory-compliant documentation |
2: Impurity Profiling with GC-MS Analysis for Pharmaceuticals
Impurity profiling using GC-MS analysis for pharmaceuticals identifies, characterizes, and quantifies all impurities in drug substances and products to ensure patient safety. Regulatory agencies require comprehensive impurity profiles for all pharmaceutical products, making this a critical quality control function.
Our laboratory performs comprehensive impurity profiling through validated GC-MS Analysis Services:
https://resolvemass.ca/gcms-analysis-service-2/
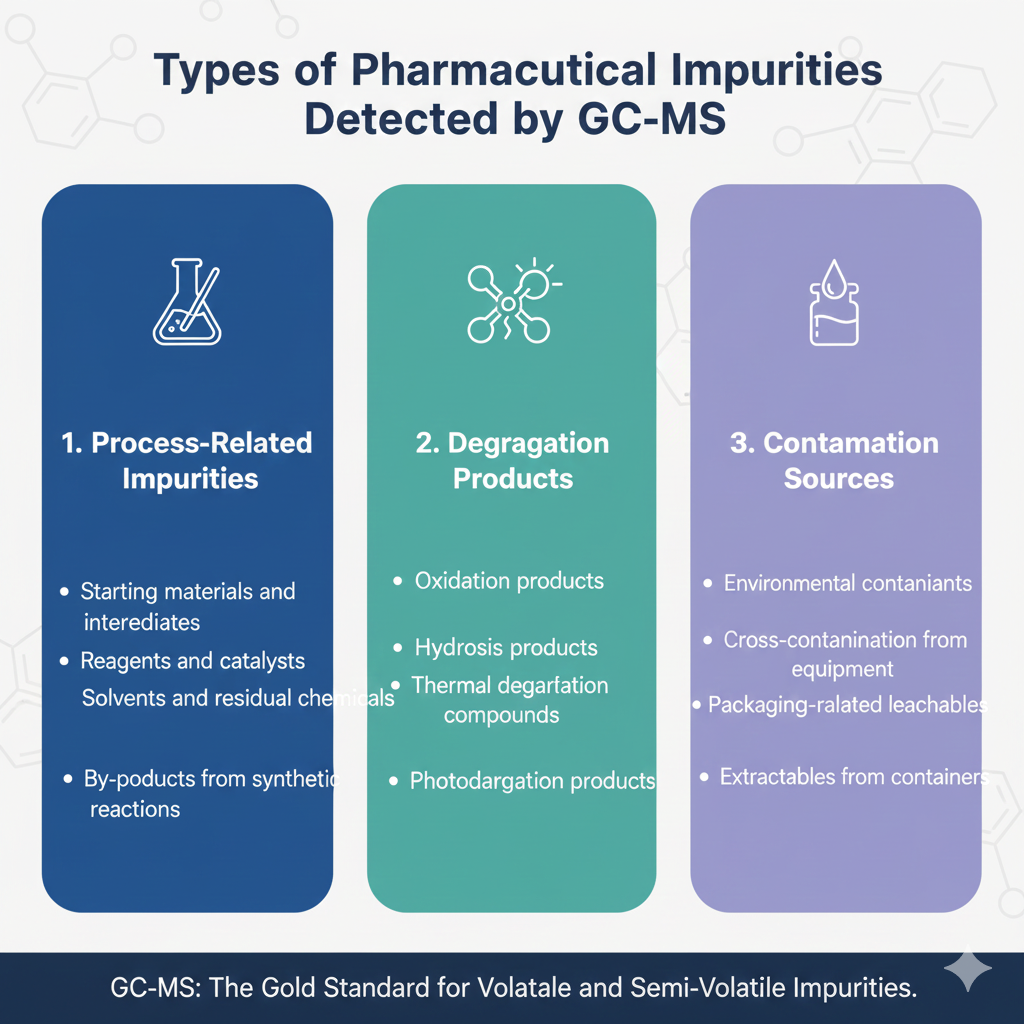
Types of Pharmaceutical Impurities Detected by GC-MS
- Process-Related Impurities
- Starting materials and intermediates
- Reagents and catalysts
- Solvents and residual chemicals
- By-products from synthetic reactions
- Degradation Products
- Oxidation products
- Hydrolysis products
- Thermal degradation compounds
- Photodegradation products
- Contamination Sources
- Environmental contaminants
- Cross-contamination from equipment
- Packaging-related leachables
- Extractables from containers
Custom Method Development for Impurity Profiling
Our approach to GC-MS analysis for pharmaceuticals begins with understanding the unique chemical properties of each drug substance. We develop customized methods that:
- Optimize separation: Column selection, temperature programming, and carrier gas flow
- Maximize sensitivity: Injection techniques, detector parameters, and sample preparation
- Ensure specificity: Mass spectral libraries, retention time locking, and confirmation ions
- Meet regulatory requirements: ICH Q3A/Q3B guidelines for impurity thresholds
For customized impurity methods and non-compendial challenges, learn more about our GC-MS Method Development Services:
https://resolvemass.ca/gcms-method-development-service/
and advanced GC-MS Method Development capabilities:
https://resolvemass.ca/gc-ms-method-development/
Quantification and Reporting Standards
ResolveMass Laboratories Inc. employs validated quantification methods that provide:
- Accurate concentration determination: Using internal standards and calibration curves
- Identification confidence: Match factors >800 for library searches, confirmation with reference standards
- Reporting limits: Typically 0.01-0.05% for specified impurities, lower for known toxic impurities
- Documentation: Full regulatory-compliant reports with method validation parameters
3: Forced Degradation Studies Using GC-MS Analysis for Pharmaceuticals
Forced degradation studies expose pharmaceutical compounds to stress conditions to identify potential degradation pathways and develop stability-indicating methods. These studies are essential for understanding how drugs degrade under various environmental conditions and for supporting regulatory submissions.
Regulatory Framework for Forced Degradation
The ICH Q1A and Q1B guidelines recommend forced degradation studies to:
- Demonstrate specificity of stability-indicating methods
- Identify potential degradation products
- Establish degradation pathways
- Support formulation development
- Provide information for package selection
Stress Conditions for Forced Degradation Studies
| Stress Condition | Typical Parameters | GC-MS Application |
|---|---|---|
| Thermal | 40-80°C for 1-4 weeks | Identifies thermolabile compounds and thermal degradation products |
| Oxidative | H₂O₂ (3-30%) at room temperature | Detects oxidation-prone functional groups and peroxide-related degradation |
| Hydrolytic (Acid) | 0.1-1N HCl at various temperatures | Reveals acid-catalyzed degradation pathways |
| Hydrolytic (Base) | 0.1-1N NaOH at various temperatures | Identifies base-labile bonds and hydrolysis products |
| Photolytic | ICH Q1B light exposure (1.2M lux-hr visible, 200 W-hr/m² UV) | Determines photostability and light-induced degradation |
Custom GC-MS Analysis for Degradation Product Identification
Our GC-MS analysis for pharmaceuticals during forced degradation studies provides comprehensive structural information for unknown degradation products. This capability is critical for:
- Peak purity assessment: Ensuring chromatographic peaks represent single compounds
- Mass spectral interpretation: Determining molecular weights and fragmentation patterns
- Structural elucidation: Proposing degradation mechanisms based on mass spectral data
- Degradation pathway mapping: Understanding how drugs break down under stress
Method Validation for Forced Degradation
ResolveMass Laboratories Inc. ensures that GC-MS methods used for forced degradation studies are:
- Stability-indicating: Capable of separating drug substance from all degradation products
- Selective: No interference from excipients, impurities, or degradation products
- Sensitive: Adequate detection limits for degradation products (typically 0.1% of label claim)
- Robust: Reproducible results across different analysts, instruments, and time periods
4: Stability Studies with GC-MS Analysis for Pharmaceuticals
Stability studies using GC-MS analysis for pharmaceuticals monitor chemical changes in drug products over time to establish shelf life and storage conditions. These studies provide evidence that pharmaceutical products maintain quality, safety, and efficacy throughout their intended storage period.
Our stability-focused GC-MS testing services are part of our broader analytical platform:
https://resolvemass.ca/gcms-analysis-service/
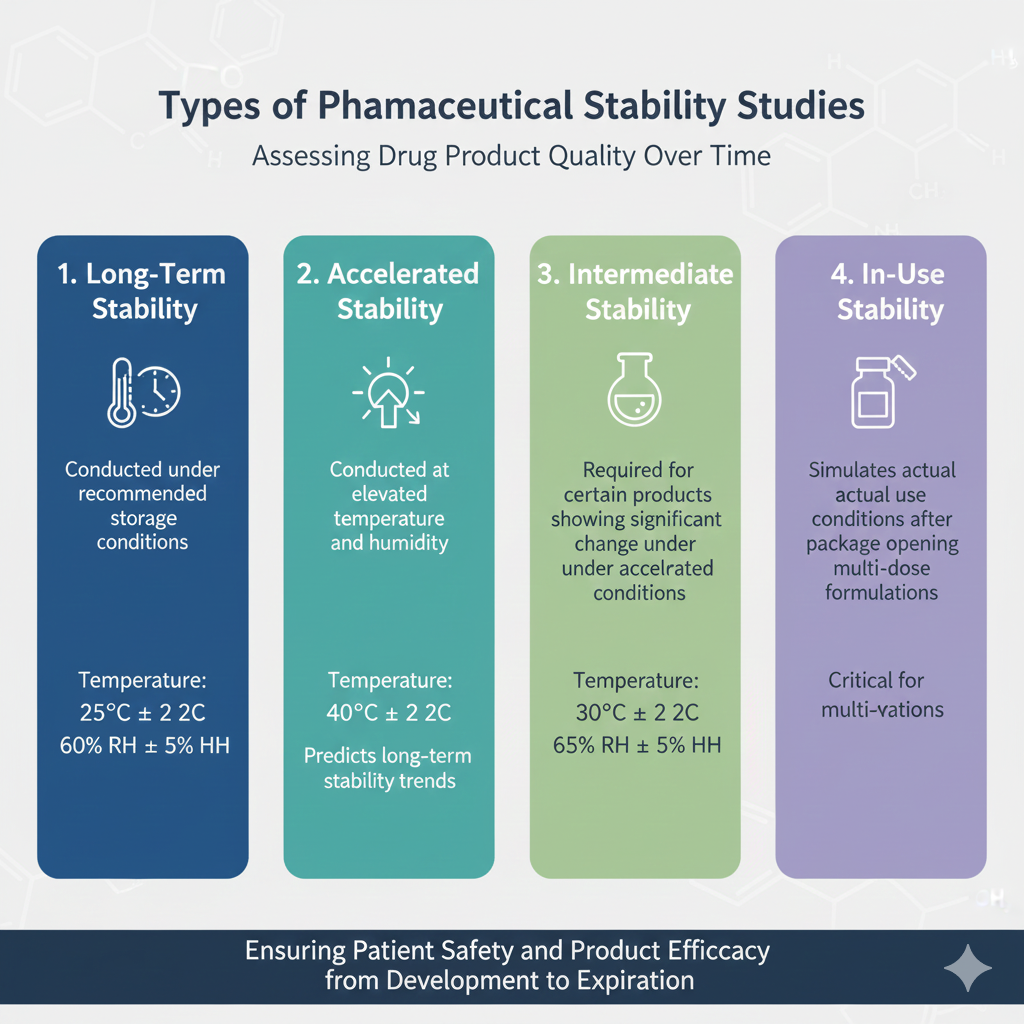
Types of Pharmaceutical Stability Studies
- Long-Term Stability
- Conducted under recommended storage conditions
- Typical duration: 12-36 months or longer
- Temperature: 25°C ± 2°C, 60% RH ± 5% RH
- Accelerated Stability
- Conducted at elevated temperature and humidity
- Duration: 6 months minimum
- Temperature: 40°C ± 2°C, 75% RH ± 5% RH
- Predicts long-term stability trends
- Intermediate Stability
- Required for certain products showing significant change under accelerated conditions
- Temperature: 30°C ± 2°C, 65% RH ± 5% RH
- In-Use Stability
- Simulates actual use conditions after package opening
- Critical for multi-dose formulations
Key Parameters Monitored by GC-MS in Stability Studies
Our custom GC-MS analysis for pharmaceuticals tracks critical quality attributes:
- Assay: Drug substance content over time
- Impurity formation: Appearance and increase of degradation products
- Total impurities: Sum of all individual impurities
- Specified impurities: Known impurities with established acceptance criteria
- Unspecified impurities: Unknown degradation products requiring identification above threshold
Stability-Indicating Method Requirements
GC-MS analysis for pharmaceuticals in stability studies must use validated stability-indicating methods that demonstrate specificity. Our methodology ensures:
- Forced degradation validation demonstrating separation of drug from degradation products
- System suitability criteria for each analytical run
- Reference standard stability monitoring
- Data trending and statistical analysis
Data Analysis and Shelf-Life Prediction
ResolveMass Laboratories Inc. provides comprehensive stability data analysis including:
- Statistical evaluation: Regression analysis, confidence intervals, and trend analysis
- Shelf-life calculation: Based on 95% confidence interval reaching specification limits
- Degradation kinetics: First-order, zero-order, or Arrhenius modeling
- Retest date establishment: For drug substances and intermediates
- Regulatory documentation: ICH-compliant stability reports and summaries
5: Advantages of Custom GC-MS Analysis Over Standard Methods
Custom GC-MS analysis for pharmaceuticals offers significant advantages over standard compendial methods by addressing unique analytical challenges. While USP, EP, and JP provide general methods, custom approaches deliver:
Tailored Analytical Solutions
- Method optimization: Customized for specific compound chemistry and matrix effects
- Enhanced sensitivity: Achieving lower detection limits when required for toxic impurities
- Improved selectivity: Separating critical pairs that co-elute in standard methods
- Faster turnaround: Optimized run times without sacrificing separation quality
Advanced Problem-Solving Capabilities
Our GC-MS analysis for pharmaceuticals addresses complex scenarios:
- Novel drug substances: No existing analytical methods available
- Complex formulations: Multiple active ingredients or challenging matrices
- Unknown impurities: Requiring identification and structural characterization
- Atypical degradation: Unexpected degradation products not covered by standard methods
- Ultra-trace analysis: Detection requirements below typical method capabilities
6: ResolveMass Laboratories Inc. Expertise in Pharmaceutical GC-MS
ResolveMass Laboratories Inc. combines state-of-the-art GC-MS instrumentation with decades of pharmaceutical analytical expertise to deliver unparalleled service quality. Our laboratory specializes in custom method development for the most challenging pharmaceutical applications.
. Our GC-MS expertise also extends to other regulated applications such as Pesticide Testing Using GC-MS in Canada:
https://resolvemass.ca/pesticide-testing-services-using-gc-ms-in-canada/
We also support complex natural matrices, including GC-MS Analysis of Plant Extracts:
https://resolvemass.ca/gcms-analysis-of-plant-extract/
Our Comprehensive Service Offerings
Impurity Analysis Services
- Complete impurity profiling for drug substances and products
- Unknown impurity identification and structural characterization
- Genotoxic impurity testing below ICH M7 threshold levels
- Residual solvent analysis per ICH Q3C guidelines
Degradation Study Services
- ICH-compliant forced degradation study design and execution
- Degradation product identification using high-resolution mass spectrometry
- Degradation pathway elucidation and mechanism studies
- Stability-indicating method development and validation
Stability Testing Services
- Long-term, accelerated, and intermediate stability programs
- ICH-compliant stability protocols and documentation
- Real-time stability monitoring with data trending
- Photostability testing per ICH Q1B requirements
Quality Assurance and Regulatory Compliance
Our GC-MS analysis for pharmaceuticals meets the highest quality standards:
- Accreditations: ISO/IEC 17025 accredited testing laboratory
- Regulatory expertise: FDA, EMA, ICH guideline compliance
- Validation protocols: Full method validation per ICH Q2(R1) requirements
- Documentation: 21 CFR Part 11 compliant electronic records
- Quality systems: Comprehensive QA/QC procedures and audits
Advanced Instrumentation Platform
ResolveMass Laboratories Inc. maintains cutting-edge GC-MS technology:
- High-resolution mass spectrometry: For accurate mass determination and elemental composition
- Triple quadrupole systems: For targeted quantification with maximum sensitivity
- Headspace and thermal desorption: For volatile compound analysis
- Automated sample preparation: Ensuring reproducibility and high throughput
- Spectral libraries: Comprehensive databases including pharmaceutical impurities
7: Industry Applications and Case Studies
Our GC-MS analysis for pharmaceuticals has supported hundreds of successful regulatory submissions across diverse therapeutic areas. While maintaining client confidentiality, we can share representative examples of our problem-solving capabilities.
Our GC-MS analysis for pharmaceuticals has supported successful INDs, ANDAs, and global regulatory submissions. For guidance on selecting the right analytical partner, read our comparison guide:
https://resolvemass.ca/best-gc-ms-analysis-services-in-north-america-how-to-compare-labs-for-accuracy-and-turnaround-time/
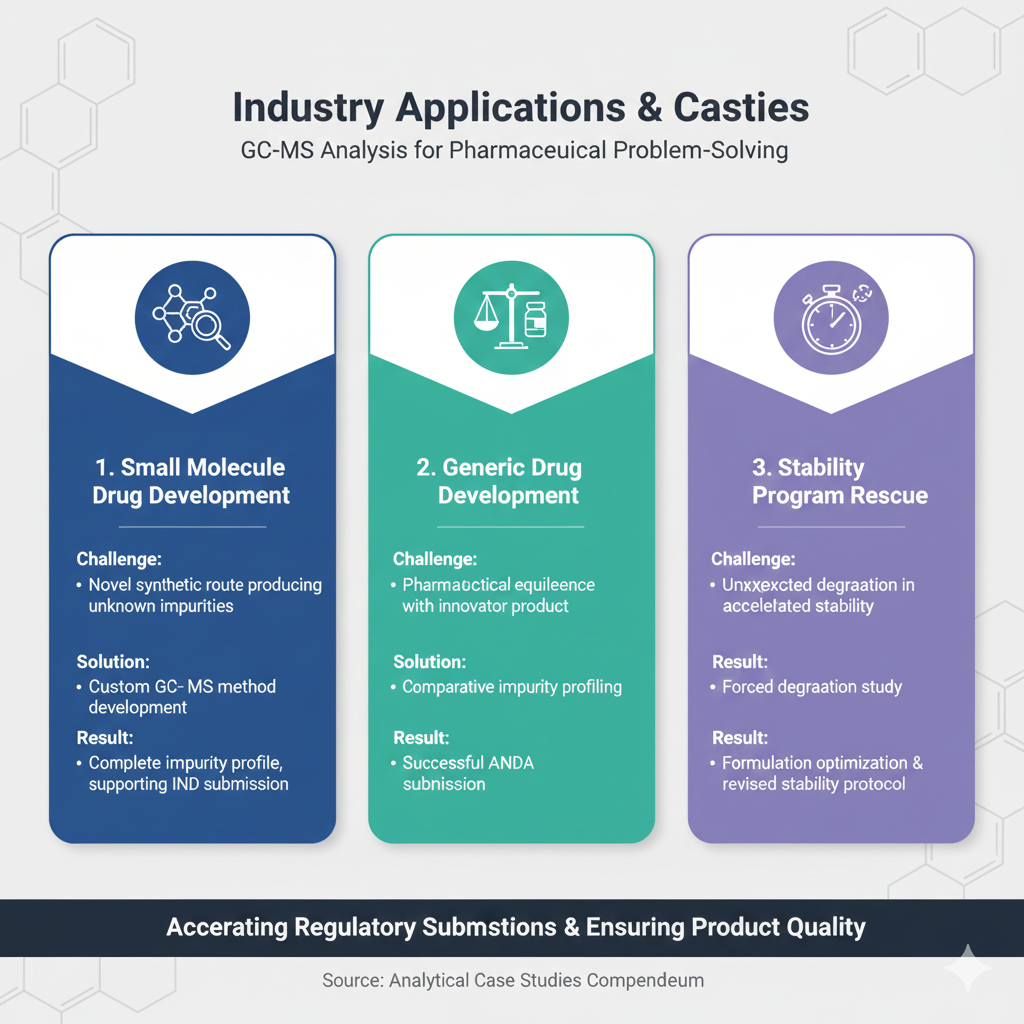
Small Molecule Drug Development
- Challenge: Novel synthetic route producing unknown impurities not covered by compendial methods
- Solution: Custom GC-MS method development with high-resolution MS identification
- Result: Complete impurity profile established, supporting IND submission
Generic Drug Development
- Challenge: Demonstrating pharmaceutical equivalence with innovator product impurity profile
- Solution: Comparative impurity profiling using optimized GC-MS analysis
- Result: Successful ANDA submission with impurity qualification data
Stability Program Rescue
- Challenge: Unexpected degradation observed in accelerated stability, threatening product launch
- Solution: Comprehensive forced degradation study and degradation pathway identification
- Result: Formulation optimization and revised stability protocol ensuring product approval
8: Choosing the Right Analytical Partner for GC-MS Analysis
Selecting an analytical laboratory for GC-MS analysis for pharmaceuticals requires careful evaluation of technical capabilities, regulatory experience, and service quality. Consider these critical factors:
Technical Competency Indicators
- Instrumentation diversity and maintenance standards
- Method development and validation experience
- Problem-solving track record for complex impurity challenges
- Scientific staff qualifications and training programs
Regulatory Track Record
- History of successful regulatory submissions
- Inspection readiness and audit history
- Understanding of global regulatory requirements
- Documentation quality and completeness
Service Excellence Markers
- Communication responsiveness and technical support
- Project timeline reliability
- Data quality and scientific rigor
- Flexibility for rush testing and custom protocols
Pharmaceutical companies evaluating GC-MS laboratories should consider technical expertise, regulatory track record, and data quality. Learn why clients choose ResolveMass in Montreal, Canada:
https://resolvemass.ca/gcms-analysis-in-montreal-canada-why-resolvemass-laboratories-inc-is-your-best-choice/
9: Future Trends in GC-MS Analysis for Pharmaceuticals
The field of GC-MS analysis for pharmaceuticals continues advancing with emerging technologies and regulatory expectations. Key trends include:
Technological Advancements
- High-resolution accurate mass spectrometry: Improving unknown identification capabilities
- Two-dimensional GC-MS: Enhanced separation for complex matrices
- Automation and robotics: Increasing throughput and reducing human error
- Data science integration: AI-assisted spectral interpretation and impurity prediction
Regulatory Evolution
- Enhanced impurity requirements: Lower reporting thresholds for mutagenic impurities
- Nitrosamine testing: Mandatory screening for N-nitrosamine impurities
- Elemental impurity monitoring: Combining GC-MS with ICP-MS approaches
- Real-time release testing: Moving toward continuous manufacturing support
Conclusion
Custom GC-MS analysis for pharmaceuticals represents the cornerstone of pharmaceutical quality assurance, enabling comprehensive impurity profiling, forced degradation studies, and stability monitoring. As pharmaceutical products become increasingly complex and regulatory standards continue rising, the need for specialized analytical expertise grows correspondingly.
ResolveMass Laboratories Inc. stands ready to partner with pharmaceutical companies at every stage of drug development and commercialization. Our custom GC-MS analysis for pharmaceuticals delivers the precision, reliability, and regulatory compliance required for today’s demanding pharmaceutical landscape. From initial impurity screening through final stability testing, our analytical capabilities support your commitment to patient safety and product quality.
Whether you’re developing novel therapeutics, qualifying generic formulations, or troubleshooting stability challenges, our team brings the technical expertise and regulatory knowledge to move your projects forward confidently. Our GC-MS analysis for pharmaceuticals has supported hundreds of successful regulatory submissions, and we’re committed to maintaining the highest standards of analytical excellence.
The pharmaceutical industry demands analytical partners who understand both the science and the regulatory landscape. At ResolveMass Laboratories Inc., we combine state-of-the-art instrumentation with deep pharmaceutical expertise to deliver analytical solutions that meet your specific needs.
Are you looking for reliable GC-MS Analysis for Pharmaceuticals?
Explore our full service offering here:
https://resolvemass.ca/gcms-analysis-service/
FAQs on GC-MS Analysis:
GC-MS impurity profiling is an analytical approach used to identify, characterize, and quantify volatile and semi-volatile impurities present in drug substances and drug products. These impurities may arise from raw materials, synthesis pathways, residual solvents, degradation reactions, or packaging interactions. GC-MS combines gas chromatographic separation with mass spectral identification, enabling structural elucidation of unknown impurities, which is critical for regulatory submissions and patient safety.
GC-MS is particularly suitable for:
-Residual solvents (ICH Q3C)
-Genotoxic and mutagenic impurities
-Volatile degradation products
-Low-molecular-weight process impurities
-Extractables and leachables
-Nitrosamines and related volatile contaminants
Non-volatile or thermally labile compounds are typically analyzed using LC-MS, while GC-MS excels where volatility and high sensitivity are required.
In forced degradation studies, GC-MS helps detect and identify volatile degradation products formed under stress conditions such as heat, light, oxidation, acid/base hydrolysis, and humidity. GC-MS provides mass spectral fingerprints that allow scientists to:
-Understand degradation pathways
-Confirm degradation mechanisms
-Differentiate between process-related and stress-induced impurities
This data supports stability-indicating method development and regulatory compliance.
During stability studies, APIs and formulations may generate volatile degradation products over time. GC-MS enables sensitive monitoring of these changes under ICH storage conditions. It helps ensure:
-Product quality throughout shelf life
-Identification of unexpected degradation compounds
-Support for shelf-life assignment and labeling claims
GC-MS data strengthens long-term stability justifications in regulatory dossiers.
Custom GC-MS method development involves tailoring sample preparation, column selection, temperature programming, ionization mode, and detection parameters to a specific API or formulation. It is necessary because:
-Each pharmaceutical matrix behaves differently
-Target impurities may be present at trace levels
-Regulatory requirements demand validated, robust methods
A customized approach ensures accuracy, reproducibility, and regulatory acceptance.
Modern GC-MS systems can detect impurities at ppm to ppb levels, depending on the compound and method design. Techniques such as:
-Headspace GC-MS
-Selective ion monitoring (SIM)
-GC-HRMS
enhance sensitivity, making GC-MS suitable for trace-level risk assessment.
Reference
- Smriti RANJAN MAJI,Chaitali ROY1, Sandip KUMAR SINHA.Gas chromatography–mass spectrometry (GC-MS): a comprehensive review of
synergistic combinations and their applications in the past two decades.https://revues.imist.ma/index.php/JASAB/article/download/40209/21702 - What is Gas Chromatography & Mass Spectrometry (GC-MS)?https://www.aimil.com/blog/gas-chromatography/

