
INTRODUCTION
GC-MS sample preparation is the critical foundation that determines the success or failure of your analytical results. While modern gas chromatography–mass spectrometry (GC-MS) instruments offer exceptional sensitivity and selectivity, studies consistently show that nearly 60–80% of analytical errors originate during sample preparation rather than instrument performance.
At ResolveMass Laboratories Inc., we have seen firsthand how scientifically optimized sample preparation enhances sensitivity, improves reproducibility, and ensures regulatory compliance. A strong understanding of the working principle of GC-MS—and how it compares with GC-MS vs GC-MS/MS—is essential before selecting the most appropriate preparation strategy.
This article explains how sample preparation for GC-MS techniques such as purge-and-trap, headspace sampling, derivatization, and extraction methods influence final results across a wide range of applications of GC-MS.
SUMMARY: HOW SAMPLE PREPARATION AFFECTS GC-MS RESULTS
- GC-MS preparation is one of the most critical determinants of analytical accuracy, sensitivity, and reproducibility.
- Techniques such as purge-and-trap, headspace, and derivatization directly influence detection limits, matrix interference, and instrument health.
- Selecting the right GC-MS sample preparation approach depends on analyte volatility, polarity, matrix complexity, and regulatory expectations.
- Poor GC-MS sample preparation can lead to analyte loss, misidentification, contamination, and regulatory non-compliance.
1. WHAT IS GC-MS SAMPLE PREPARATION?
GC-MS sample refers to all processes used to convert a raw sample into a form compatible with gas chromatography and mass spectrometric detection. In practical terms, it determines which compounds enter the GC-MS system, at what concentration, and with how much matrix interference.
This step is especially critical in regulated testing environments such as GC-MS analysis for pharmaceuticals, polymers, and environmental samples.
Key objectives of GC-MS sample preparation include:
- Isolating target analytes from complex matrices
- Improving analyte volatility and thermal stability
- Reducing matrix-related interferences
- Enhancing sensitivity and detection limits
- Protecting GC-MS instrumentation
Without proper sample preparation, even the most advanced instruments cannot deliver accurate or reproducible results.
2. HOW SAMPLE PREPARATION DIRECTLY AFFECTS GC-MS RESULTS
GC-MS preparation affects analytical results within the first seconds of analysis. The selected preparation technique directly influences peak shape, retention time stability, mass spectral clarity, and quantitative accuracy.
This impact is especially evident in regulated and trace-level testing such as pesticide testing using GC-MS in Canada.
Direct impacts of GC-MS sample preparation include:
- Sensitivity: Pre-concentration techniques improve trace-level detection
- Selectivity: Cleaner extracts reduce co-elution and spectral overlap
- Reproducibility: Controlled preparation minimizes variability
- Accuracy: Reduced analyte loss improves quantitation
- Instrument performance: Cleaner samples extend column and ion source life
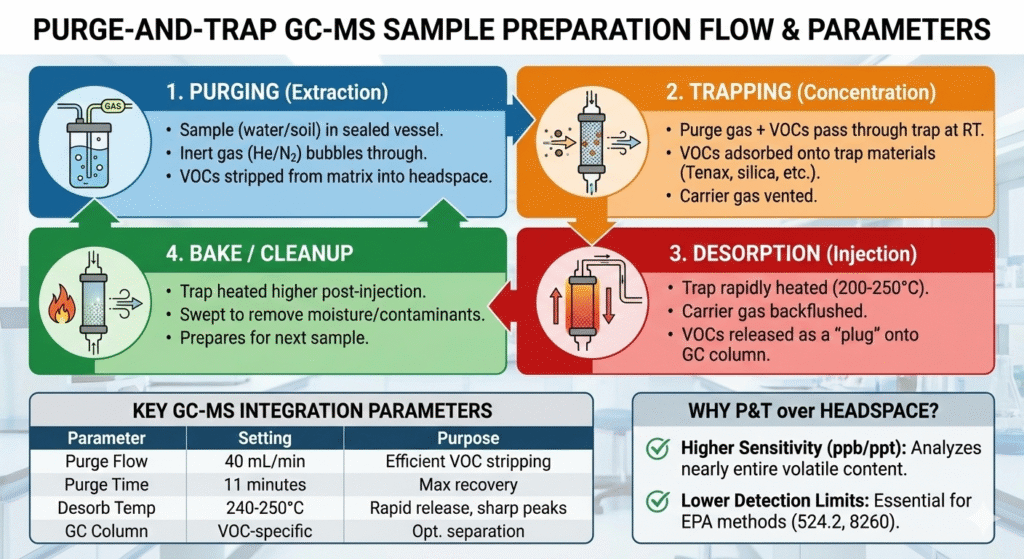
3. PURGE-AND-TRAP GC-MS SAMPLE PREPARATION
Purge-and-trap sample preparation is one of the most sensitive techniques available for volatile organic compound (VOC) analysis.
In this approach, volatile analytes are purged from a liquid or solid matrix using an inert gas and captured on a sorbent trap before thermal desorption into the GC-MS. This technique is widely used in GC-MS residual solvent analysis and regulatory-driven workflows supported by professional GC-MS analysis services.
WHEN SHOULD PURGE-AND-TRAP BE USED?
Purge-and-trap is ideal when ultra-low detection limits are required.
Common applications include:
- Environmental VOC analysis
- Residual solvent testing
- Drinking water analysis
- Extractables and leachables studies
IMPACT ON GC-MS RESULTS
- Sensitivity: Very high (ppt–ppb range)
- Matrix interference: Minimal
- Reproducibility: High when optimized
- Instrument contamination: Low
While purge-and-trap dramatically improves sensitivity, careful optimization is required to avoid water carryover or artifact formation.
4. HEADSPACE GC-MS SAMPLE PREPARATION
Headspace sample preparation analyzes volatile compounds present in the vapor phase above a sample rather than injecting the sample directly. This approach is central to routine GC-MS analysis services dealing with complex matrices.
This technique answers a critical question upfront: how can volatile analytes be isolated while leaving non-volatile matrix components behind?
STATIC VS DYNAMIC HEADSPACE
- Static headspace: Vapor analyzed at equilibrium; simple and highly reproducible
- Dynamic headspace: Continuous purging; higher sensitivity for trace compounds
BENEFITS FOR GC-MS RESULTS
- Reduced matrix effects
- Cleaner chromatograms
- Improved method robustness
- Lower instrument maintenance
Headspace sample preparation is widely used in pharmaceutical testing, polymer analysis, and GC-MS testing for medical devices.

5. DERIVATIZATION IN GC-MS SAMPLE PREPARATION
Derivatization is required when analytes are polar, non-volatile, or thermally unstable and cannot be analyzed directly by GC-MS.
In derivatization-based sample preparation, functional groups are chemically modified to improve volatility, thermal stability, and chromatographic performance. These workflows are typically developed through expert GC-MS method development and GC-MS method development services.
COMMON DERIVATIZATION TARGETS
- Organic acids
- Alcohols and phenols
- Amines
- Sugars and metabolites
HOW DERIVATIZATION AFFECTS GC-MS RESULTS
- Improved peak shape and resolution
- Enhanced thermal stability
- Altered mass spectral fragmentation patterns
While derivatization enhances detectability, it requires validated procedures and derivatized reference standards to maintain quantitative accuracy.
6. OTHER IMPORTANT GC-MS SAMPLE PREPARATION TECHNIQUES
Beyond purge-and-trap, headspace, and derivatization, additional sample preparation techniques are commonly applied in advanced laboratories operating across the United States and Montreal:
- Solid-phase microextraction (SPME): Solvent-free, selective extraction
- Liquid–liquid extraction (LLE): Traditional but matrix-dependent
- Solid-phase extraction (SPE): Enhanced cleanup and selectivity
Each technique affects GC-MS results differently and must be selected based on analyte chemistry and matrix complexity.

7. MATRIX EFFECTS AND THEIR ROLE IN GC-MS SAMPLE PREPARATION
Matrix interference remains one of the biggest challenges in GC-MS analysis. Improper sample preparation can result in:
- Suppressed analyte signals
- Co-eluting peaks
- Contaminated ion sources
- Reduced method robustness
Accurate identification and quantitation often require advanced expertise in GC-MS chromatogram interpretation to distinguish true analytes from matrix artifacts.
8. REGULATORY CONSIDERATIONS FOR GC-MS SAMPLE PREPARATION
Regulatory agencies expect GC-MS sample methods to be scientifically justified and properly validated.
Key guidelines influencing GC-MS sample preparation include:
- USP and EP chapters
- ICH Q2 and Q3
- EPA analytical methods
Using guideline-aligned preparation strategies supports data acceptance and smoother submissions, particularly when working with laboratories recognized among the best GC-MS analysis services in North America.
9. HOW TO SELECT THE RIGHT GC-MS SAMPLE PREPARATION METHOD
The correct GC-MS sample method depends on multiple factors:
- Analyte volatility and polarity
- Matrix complexity
- Required detection limits
- Regulatory expectations
- Instrument protection
No single sample preparation approach fits all applications; method development expertise is essential.

CONCLUSION: GC-MS SAMPLE PREPARATION DEFINES DATA QUALITY
GC-MS sample preparation is not a preliminary step—it defines the quality, accuracy, and credibility of analytical results. Techniques such as purge-and-trap, headspace, and derivatization directly influence sensitivity, selectivity, and reproducibility.
By applying scientifically justified sample preparation strategies, laboratories can generate reliable, regulator-ready data while protecting instrumentation and minimizing rework.
FAQs on Sample Preparation Affects GC-MS Results
GC-MS sample preparation involves extraction, purification, and concentration to get volatile analytes into a suitable volatile solvent (like hexane, DCM) in a clean glass vial, free of particles, water, acids, or bases, often using techniques like SPE, filtration, or derivatization, to ensure accurate GC injection and protect the instrument.
Sample preparation for mass spectrometry is used for the optimization of a sample for analysis in a mass spectrometer (MS). Each ionization method has certain factors that must be considered for that method to be successful, such as volume, concentration, sample phase, and composition of the analyte solution.
-Sample preparation: Analyte loss, evaporation, contamination, poor derivatization
-Injection: Incorrect volume, split ratio errors, inlet discrimination
-Column: Wrong column choice, contamination, aging, overloading
-Carrier gas/flow: Leaks, unstable flow, low gas purity
-Temperature control: Inaccurate oven, inlet, or detector temperatures
-Detector: Contamination, poor sensitivity, signal drift
-Calibration: Incorrect standards, response factor drift
-Data processing: Peak integration and identification errors
The sample is injected in the GC where the sample is vaporized. It is then separated in the column based on the compounds boiling points and chemical interaction with the stationary phase.
GC/MS methods usually require 1-3 mL of sample. Because of the higher sample volume needed for GC/MS analysis, higher mass sorbent beds in the extraction cartridge are usually needed as well. The larger sorbent bed allows for a higher amount of the compounds of interest to bind to the sorbent bed.
Reference
- Sample Preparation Techniques for Gas Chromatography.https://books.google.com/books?hl=en&lr=&id=nQj8DwAAQBAJ&oi=fnd&pg=PA45&dq=How+Sample+Preparation+Affects+GC-MS+Results:+Purge-and-Trap,+Headspace,+Derivatization+%26+More&ots=14sdgJrmsn&sig=_O6u-Kg3LcG2Eo3uYUz5_YhROBE
- Modern Methods of Sample Preparation for GC Analysis.https://link.springer.com/article/10.1365/s10337-008-0937-3
- Sample Preparation Techniques for GC.https://link.springer.com/chapter/10.1007/978-3-642-54640-2_16
- Headspace Techniques for Volatile Sampling.https://www.sciencedirect.com/science/chapter/handbook/abs/pii/S0166526X17300090
- Gas chromatography and GC/MS.Progress in Pharmaceutical and Biomedical Analysis.https://www.sciencedirect.com/science/chapter/bookseries/abs/pii/S1464345600800253

