Introduction
Understanding GC-MS method validation cost is critical for pharmaceutical, biotech, and CRO organizations planning analytical development projects. Method validation represents a significant investment in ensuring analytical procedures meet stringent regulatory requirements while providing reliable, accurate data for critical business decisions.For foundational insights into the technique, see Gas Chromatography–Mass Spectrometry: https://resolvemass.ca/gas-chromatography-mass-spectrometry/
GC-MS method validation cost encompasses far more than laboratory analysis fees. A comprehensive validation project includes method development optimization, validation parameter testing, statistical analysis, regulatory-compliant documentation, and quality review processes. For organizations seeking FDA, EMA, or Health Canada approval, understanding these cost components enables accurate project planning and prevents budget overruns that could delay critical product launches.
To understand how GC-MS is applied across different industries and services, you may find these resources helpful:
- Applications of GC-MS: https://resolvemass.ca/applications-of-gcms/
- Working principle of GC-MS: https://resolvemass.ca/working-principle-of-gc-ms/
- Best GC-MS analysis services in North America: https://resolvemass.ca/best-gc-ms-analysis-services-in-north-america-how-to-compare-labs-for-accuracy-and-turnaround-time/
At ResolveMass Laboratories Inc., we’ve conducted hundreds of GC-MS method validations across pharmaceutical, biotech, and contract research settings. Our extensive experience reveals that transparency about GC-MS method validation cost helps clients make informed decisions while avoiding common pitfalls that inflate project expenses. This comprehensive guide breaks down all cost factors, provides realistic budget ranges, and offers practical strategies for optimizing your validation investment without compromising quality or regulatory compliance.
Whether you’re validating methods for drug substance release testing, impurity analysis, residual solvent determination, or environmental/pesticide testing, understanding the true GC-MS method validation cost ensures accurate budgeting and efficient project execution. Key services referenced in this article include:
- GC-MS method development service: https://resolvemass.ca/gcms-method-development-service/
- GC-MS analysis in the United States: https://resolvemass.ca/gcms-analysis-in-united-states/
- GC-MS analysis for pharmaceutical samples: https://resolvemass.ca/gc-ms-analysis-for-pharmaceuticals/
- GC-MS residual solvent analysis: https://resolvemass.ca/gcms-residual-solvent-analysis-what-you-must-know/
- Pesticide testing via GC-MS in Canada: https://resolvemass.ca/pesticide-testing-services-using-gc-ms-in-canada/
- GC-MS analysis of plant extract: https://resolvemass.ca/gcms-analysis-of-plant-extract/
- GC-MS method development: https://resolvemass.ca/gc-ms-method-development/
Summary
Understanding GC-MS method validation cost is essential for accurate project budgeting in pharmaceutical, biotech, and CRO settings. This comprehensive guide reveals:
- Typical Cost Range: GC-MS method validation cost typically ranges from $8,000 to $35,000+ depending on complexity, with pharmaceutical projects averaging $15,000-$25,000
- Key Cost Drivers: Complexity level, validation parameters required, timeline urgency, regulatory requirements, and matrix complexity directly impact total investment
- Industry Variations: Pharmaceutical validation costs 20-40% higher than biotech due to stricter regulatory documentation; CRO projects vary widely based on client specifications
- Cost Components: Laboratory analysis (40-50%), materials and reference standards (15-25%), documentation and reporting (20-30%), and project management (10-15%)
- Budget Optimization: Strategic planning, phased validation approaches, and leveraging existing data can reduce GC-MS method validation cost by 15-30% without compromising quality
For details on how ResolveMass can assist with GC-MS method validation specifically in Montreal, view: https://resolvemass.ca/gcms-analysis-in-montreal-canada-why-resolvemass-laboratories-inc-is-your-best-choice/
1: What Factors Determine GC-MS Method Validation Cost?
GC-MS method validation cost varies based on method complexity, validation scope, regulatory requirements, timeline constraints, and matrix characteristics. Understanding these variables enables accurate budget estimation.
Primary Cost Drivers
1. Method Complexity Level
| Complexity Tier | Characteristics | Typical Cost Impact |
|---|---|---|
| Simple | Single analyte, clean matrix, established conditions | Baseline cost |
| Moderate | 2-5 analytes, some matrix interference, standard validation | +30-50% above baseline |
| Complex | Multiple analytes, challenging separation, derivatization required | +80-120% above baseline |
| Highly Complex | >10 analytes, severe matrix effects, novel methodology | +150-250% above baseline |
2. Validation Parameters Required
The scope of validation testing significantly impacts GC-MS method validation cost:
- Basic parameters (specificity, linearity, accuracy, precision): Foundation cost
- Extended parameters (robustness, intermediate precision): +20-30%
- Comprehensive parameters (ruggedness, system suitability, stability): +40-60%
- Specialized testing (carryover, dilution integrity, container closure): +15-25% each
3. Regulatory Requirements
- FDA submission quality: Requires extensive documentation, audit trails, QA review
- ICH guideline compliance: Formal protocols, statistical analysis, comprehensive reports
- GLP/GMP compliance: Enhanced quality systems, training records, equipment qualification
- Multiple regulatory regions: Documentation for FDA, EMA, Health Canada simultaneously
4. Timeline Urgency
Rush timelines directly increase GC-MS method validation cost:
- Standard timeline (6-8 weeks): Baseline pricing
- Expedited (3-4 weeks): +25-35% premium
- Rush (2 weeks or less): +50-75% premium
- Emergency turnaround: Custom pricing, potentially +100%
5. Sample Matrix Complexity
- Clean standards in solvent: Baseline
- Pharmaceutical formulations: +15-25%
- Biological matrices (plasma, tissue): +40-60%
- Complex formulations (emulsions, suspensions): +30-50%
- Environmental or food matrices: +50-80%
Learn more about the foundational analytical techniques that underpin these validations at: https://resolvemass.ca/gcms-analysis-service/
2: Typical GC-MS Method Validation Cost Ranges by Industry
GC-MS method validation cost varies significantly across pharmaceutical, biotech, and CRO sectors due to different regulatory expectations, documentation requirements, and quality standards. Understanding industry-specific pricing helps set realistic budgets.
Pharmaceutical Industry GC-MS Method Validation Cost
Pharmaceutical validation projects typically represent the highest investment due to stringent FDA and ICH requirements. Cost ranges include:
Validation for pharmaceutical methods often has the highest cost due to strict regulatory expectations. For cost breakdowns based on application type, see:
- Residual solvent analysis with GC-MS: https://resolvemass.ca/gcms-residual-solvent-analysis-what-you-must-know/
- GC-MS method services: https://resolvemass.ca/gcms-analysis-service-2/
Standard Pharmaceutical Method Validation
- Simple single-analyte method: $10,000 – $15,000
- Multi-component drug product: $18,000 – $28,000
- Impurity profiling method: $15,000 – $25,000
- Residual solvent analysis: $12,000 – $20,000
- Complex stability-indicating method: $25,000 – $40,000
Key Cost Components in Pharma:
- Rigorous protocol review and approval processes
- Extensive documentation for regulatory submissions
- Multiple QA review cycles
- Long-term reference standard stability studies
- Comprehensive deviation investigations if needed
Biotech Industry GC-MS Method Validation Cost
Biotech method validation generally costs 20-30% less than pharmaceutical due to different regulatory pathways and documentation approaches. Typical ranges:
Biotech Validation Projects
- Process monitoring methods: $8,000 – $14,000
- Extractables/leachables analysis: $15,000 – $25,000
- Cell culture media components: $10,000 – $18,000
- Bioprocess impurities: $12,000 – $22,000
- Quality control release testing: $14,000 – $24,000
Biotech Cost Advantages:
- More flexible documentation requirements
- Faster protocol approval processes
- Focus on fitness-for-purpose rather than comprehensive validation
- Streamlined statistical analysis approaches
CRO Project GC-MS Method Validation Cost
Contract research organization validation costs vary widely based on client specifications and study type. CRO pricing typically includes:
CRO-Specific Validation
- Client method transfer and validation: $12,000 – $22,000
- Novel method development + validation: $20,000 – $35,000
- Multi-site method validation: $25,000 – $45,000
- Rapid validation for clinical trials: $15,000 – $28,000
- Regulatory submission support package: $18,000 – $32,000
CRO Pricing Considerations:
- Project management overhead included
- Regulatory expertise premium
- Quality system maintenance costs
- Potential for volume discounts on multiple methods
3: Detailed Breakdown of GC-MS Method Validation Cost Components
Understanding the specific components that comprise GC-MS method validation cost enables better budget allocation and identification of optimization opportunities. A typical validation project divides costs across several categories.
Laboratory Analysis and Testing (40-50% of Total Cost)
This represents the largest portion of GC-MS method validation cost:
Validation Parameter Testing
- Specificity testing: 8-16 injections, $1,200 – $2,500
- Linearity assessment: 5-7 concentration levels in triplicate, $2,000 – $3,500
- Accuracy studies: 9+ measurements across 3 levels, $1,800 – $3,200
- Precision testing: Repeatability and intermediate precision, $2,500 – $4,500
- Range verification: Supporting data for linearity, $800 – $1,500
- Detection/quantitation limits: Multiple dilutions and replicates, $1,000 – $2,000
- Robustness evaluation: Systematic variation of parameters, $2,500 – $5,000
Instrument Time and Operation
- GC-MS runtime and maintenance: $150-300 per hour
- Method optimization runs: 20-40 hours typical
- Validation execution: 40-80 hours depending on complexity
- Troubleshooting and re-analysis: 10-20% buffer recommended
Materials and Reference Standards (15-25% of Total Cost)
Material costs for GC-MS method validation include:
- Certified reference standards: $300 – $2,000 per compound
- Internal standards: $200 – $800 per compound
- Matrix materials: $100 – $1,500 depending on complexity
- Solvents and reagents: $300 – $800 per validation
- Consumables: Vials, septa, columns, liners, $400 – $1,200
- Quality control samples: Preparation and testing, $500 – $1,500
Cost-Saving Opportunities:
- Client-supplied reference standards (must meet quality requirements)
- Leveraging existing validated solutions
- Strategic purchasing for multi-method programs
Documentation and Reporting (20-30% of Total Cost)
Comprehensive documentation represents a significant component of GC-MS method validation cost:
Required Documentation
- Validation protocol development: 8-16 hours, $1,200 – $2,400
- Data collection and organization: 12-20 hours, $1,500 – $3,000
- Statistical analysis and interpretation: 8-16 hours, $1,200 – $2,400
- Final validation report: 16-32 hours, $2,400 – $4,800
- QA review and approval: 8-16 hours, $1,200 – $2,400
- Regulatory submission package: 8-20 hours, $1,200 – $3,000
Documentation Complexity Factors:
- FDA submission vs. internal use
- Electronic vs. paper-based systems
- Multi-region regulatory requirements
- Client-specific formatting requirements
Project Management and Quality Oversight (10-15% of Total Cost)
Professional project management ensures validation efficiency:
- Project planning and timeline development
- Resource allocation and scheduling
- Client communication and status updates
- Risk management and contingency planning
- Quality assurance oversight
- Technical review and approval processes
For detailed service options that include documentation and reporting support, reference:
https://resolvemass.ca/gcms-method-development-service/
4: Hidden Costs That Impact GC-MS Method Validation Budgets
Beyond the obvious expenses, several hidden factors can significantly increase actual GC-MS method validation cost. Awareness of these elements prevents budget surprises.
Out-of-Specification (OOS) Investigations
OOS results require formal investigation, potentially adding $2,000-$5,000 to project costs:
- Root cause analysis and documentation
- Retesting and reanalysis
- Protocol amendments if needed
- Extended QA review processes
- Timeline delays impacting other projects
Method Transfer Challenges
Transferring methods from development to validation often reveals unexpected costs:
- Equipment differences requiring method adjustment: $1,500 – $3,500
- Matrix differences needing additional testing: $1,000 – $3,000
- Reference standard sourcing issues: $500 – $2,000
- Chromatographic performance variations: $1,200 – $2,800
For deeper insights on how method development affects total cost, see:
https://resolvemass.ca/gc-ms-method-development/
Instrument Qualification and Maintenance
GC-MS instrument status impacts validation feasibility:
- Installation qualification (IQ): $2,000 – $4,000
- Operational qualification (OQ): $3,000 – $6,000
- Performance qualification (PQ): $2,500 – $5,000
- Unplanned maintenance during validation: $1,000 – $5,000
- Column replacement and conditioning: $800 – $2,000
Regulatory Amendments and Updates
Post-validation regulatory requests can add unexpected costs:
- Protocol amendments: $500 – $2,000 per revision
- Additional validation parameters: $2,000 – $5,000
- Supplemental testing for agency questions: $1,500 – $4,000
- Documentation updates and resubmission: $1,000 – $3,000
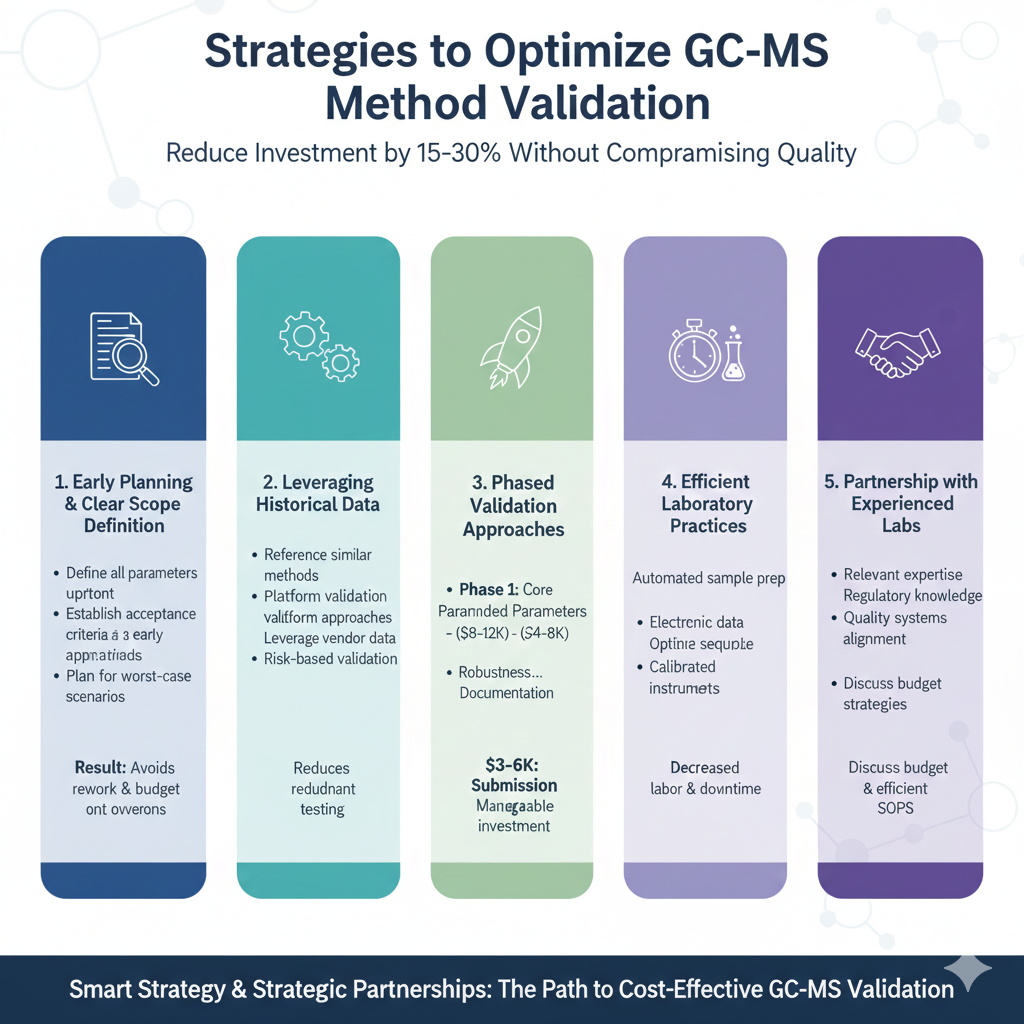
5: Strategies to Optimize GC-MS Method Validation Cost
Strategic planning and smart decision-making can reduce GC-MS method validation cost by 15-30% without compromising quality or regulatory compliance. Implement these proven approaches.
Early Planning and Clear Scope Definition
Preventing scope creep saves significant validation costs:
- Define all validation parameters upfront before protocol writing
- Establish acceptance criteria early with stakeholder agreement
- Identify regulatory requirements specific to your submission
- Plan for worst-case scenarios in initial protocol
- Build appropriate contingencies into timeline and budget
Leveraging Historical Data and Platform Approaches
Strategic use of existing information reduces redundant testing:
- Reference similar validated methods within your organization
- Use platform validation approaches for method families
- Leverage vendor data for columns and reagents appropriately
- Build on developmental data where scientifically justified
- Implement risk-based validation for low-risk applications
For an overview of GC-MS applications that might help scope planning, explore:
https://resolvemass.ca/applications-of-gcms/
Phased Validation Approaches
Staged validation reduces upfront investment while maintaining compliance:
Phase 1: Core Parameters ($8,000 – $12,000)
- Specificity, linearity, accuracy, precision
- Enables method use under development protocols
- Provides early confidence in method performance
Phase 2: Extended Parameters ($4,000 – $8,000)
- Robustness, intermediate precision, stability
- Completed prior to regulatory submission
- Allows operational use while completing full validation
Phase 3: Submission Package ($3,000 – $6,000)
- Final documentation and formatting
- Regulatory-specific requirements
- QA review and approval
Efficient Laboratory Practices
Operational efficiency directly impacts GC-MS method validation cost:
- Optimize sample sequences to maximize instrument utilization
- Use automated sample preparation where appropriate
- Implement efficient electronic data capture systems
- Schedule related validations sequentially to share setups
- Maintain well-calibrated, qualified instruments to avoid delays
Partnership with Experienced Validation Laboratories
Choosing the right analytical partner optimizes total project cost:
- Select laboratories with relevant experience in your product type
- Ensure appropriate regulatory expertise for your submission region
- Verify quality systems match your compliance requirements
- Discuss budget optimization strategies during planning
- Consider multi-project partnerships for volume efficiencies
6: Comparing In-House vs. Outsourced GC-MS Method Validation Cost
Organizations must decide whether to conduct GC-MS method validation internally or outsource to specialized laboratories. Each approach has distinct cost implications.
In-House Validation Cost Considerations
Total ownership costs for internal validation:
| Cost Category | Annual Investment | Per-Method Allocation |
|---|---|---|
| Capital Equipment | GC-MS system: $150,000 – $300,000 | Depreciation: $15,000 – $30,000 over 10 years |
| Personnel | Dedicated analyst: $80,000 – $120,000 fully loaded | $8,000 – $12,000 per validation |
| Quality Systems | Software, training, audits: $50,000 – $100,000 | $5,000 – $10,000 per validation |
| Consumables & Maintenance | Annual service, columns, reagents: $30,000 – $50,000 | $3,000 – $5,000 per validation |
| Facility Costs | Laboratory space, utilities: $40,000 – $80,000 | $4,000 – $8,000 per validation |
Break-Even Analysis:
- Organizations conducting 8+ validations annually may benefit from internal capacity
- Fewer than 5 validations per year typically favor outsourcing
- Consider utilization rates and opportunity costs
Outsourced Validation Advantages
Benefits that justify outsourcing GC-MS method validation cost:
- No capital investment: Eliminate $200,000+ equipment purchase
- Regulatory expertise: Access to specialists with submission experience
- Flexible capacity: Scale up/down based on project needs
- Faster turnaround: Dedicated resources and established processes
- Risk transfer: Laboratory assumes technical and regulatory risk
- Focus on core competencies: Internal teams concentrate on product development
Hybrid Approaches
Many organizations optimize costs through strategic combinations:
- Develop methods internally, validate externally
- Conduct routine validations in-house, complex ones outsourced
- Use CROs for overflow during peak periods
- Partner for specialized techniques (headspace, derivatization)
- Leverage external expertise for novel analytical challenges
7: GC-MS Method Validation Cost by Validation Type
Different validation types require different levels of investment based on testing scope and regulatory expectations. Understanding type-specific costs enables accurate budgeting.
Full Method Validation Cost
Comprehensive validation for new analytical methods: $15,000 – $30,000
This represents the highest GC-MS method validation cost tier, including:
- All ICH Q2(R1) parameters tested
- Comprehensive documentation package
- Full QA review and approval
- Regulatory submission-ready reports
- Extensive statistical analysis
Typical applications: New drug applications, original equipment manufacturer methods, novel analytical approaches
Partial Method Validation Cost
Modified validations for method changes: $8,000 – $18,000
Reduced scope when building on existing validated methods:
- Testing limited to parameters affected by changes
- Bridging studies to original validation
- Abbreviated documentation
- Faster approval processes
Typical applications: Equipment changes, minor methodology adjustments, reagent substitutions
Method Verification/Transfer Cost
Confirming established methods in new laboratories: $5,000 – $12,000
Lowest tier of GC-MS method validation cost:
- Abbreviated testing of critical parameters
- Comparison to original validation results
- Streamlined documentation
- Focus on equivalency demonstration
Typical applications: Multi-site implementations, CRO transfers, compendial method confirmation
Cross-Validation Cost
Comparing two analytical methods: $12,000 – $22,000
Specialized validation demonstrating equivalency:
- Side-by-side analysis of samples
- Statistical comparison of results
- Documentation of any differences
- Justification for method substitution
Typical applications: Method improvements, platform changes, regulatory agency requests
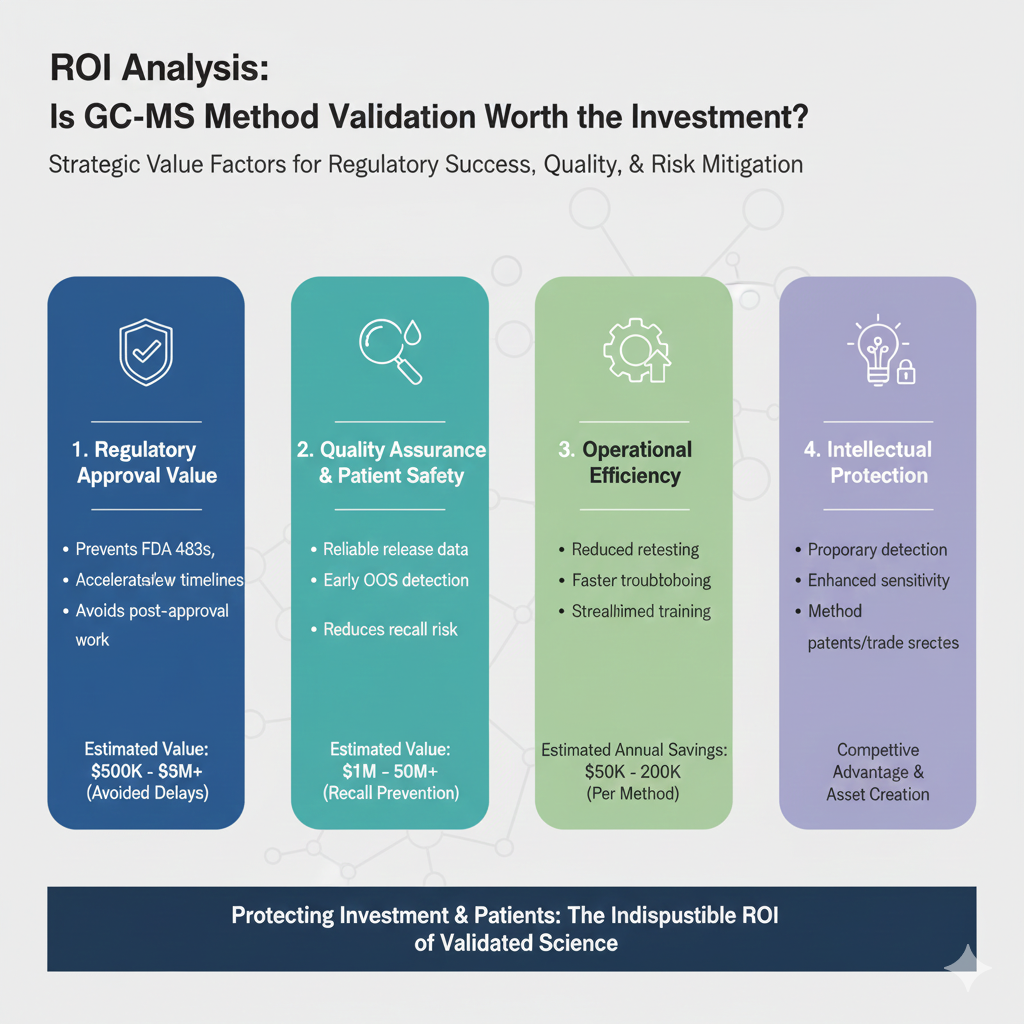
8: ROI Analysis: Is GC-MS Method Validation Worth the Investment?
While GC-MS method validation cost represents significant investment, the return on investment through regulatory success, quality assurance, and risk mitigation is substantial. Consider these value factors.
Regulatory Approval Value
A properly validated method directly impacts submission success:
- Prevents costly FDA Form 483 observations or warning letters
- Eliminates Complete Response Letters related to analytical issues
- Accelerates review timelines through complete, compliant submissions
- Avoids post-approval commitment validation work
- Estimated value: $500,000 – $5,000,000+ in avoided delays and rework
Quality Assurance Benefits
Validated methods protect product quality and patient safety:
- Reliable results for release testing decisions
- Early detection of out-of-specification batches
- Consistent data supporting trending and investigations
- Reduced risk of product recalls or field alerts
- Estimated value: Prevention of a single recall ($1M – $50M+)
Operational Efficiency
Well-validated methods improve laboratory productivity:
- Reduced retesting due to method failures
- Faster troubleshooting with established performance criteria
- Streamlined training with documented procedures
- Fewer out-of-specification investigations
- Estimated annual savings: $50,000 – $200,000 per method
Intellectual Property Protection
Validated analytical methods can represent competitive advantages:
- Proprietary detection techniques for novel compounds
- Enhanced sensitivity for difficult analytes
- Unique sample preparation approaches
- Potential for method patents or trade secrets
9: How ResolveMass Laboratories Delivers Cost-Effective GC-MS Method Validation
ResolveMass Laboratories Inc. optimizes GC-MS method validation cost through experience, efficiency, and scientific excellence. Our approach delivers maximum value for your investment.
Transparent Pricing Model
We provide detailed cost estimates including:
- Itemized quotations: Clear breakdown of all cost components
- Fixed-price options: Eliminate budget uncertainty for standard validations
- Tiered service levels: Choose the documentation level matching your needs
- Volume discounts: Reduced per-method pricing for multi-method programs
- No hidden fees: All potential costs disclosed upfront
Efficiency Through Experience
Our extensive validation portfolio reduces costs through:
- Optimized protocols based on 500+ completed validations
- Established vendor relationships for cost-effective materials
- Streamlined data systems minimizing manual effort
- Cross-trained staff enabling flexible resource allocation
- Proven troubleshooting approaches preventing delays
Quality Systems That Prevent Costly Delays
Investment in quality infrastructure protects your budget:
- ISO/IEC 17025 accredited processes
- Validated LIMS for data integrity
- Regular instrument qualification preventing mid-project failures
- Comprehensive training programs ensuring consistent execution
- Robust review processes catching issues early
Flexible Service Options
Choose the approach that optimizes your GC-MS method validation cost:
Full-Service Validation ($15,000 – $28,000)
- Complete project from protocol through report
- All materials and standards provided
- Comprehensive regulatory documentation
- Ideal for organizations without internal analytical infrastructure
Collaborative Validation ($10,000 – $18,000)
- Shared responsibilities between client and laboratory
- Client provides some materials or historical data
- Joint protocol development
- Optimizes cost when client has relevant expertise
Consultation and Review Services ($5,000 – $12,000)
- Expert review of client-generated validation data
- Protocol development and statistical analysis support
- Regulatory submission assistance
- Best for organizations with internal laboratories
Conclusion:
Understanding GC-MS method validation cost enables pharmaceutical, biotech, and CRO organizations to budget accurately while ensuring analytical methods meet regulatory requirements and business needs. While validation represents a substantial investment—typically $8,000 to $35,000 depending on complexity—the return through regulatory success, quality assurance, and operational efficiency far exceeds the initial expenditure.
The key to optimizing GC-MS method validation cost lies in early planning, clear scope definition, and partnership with experienced analytical laboratories. Whether you choose in-house validation, complete outsourcing, or a hybrid approach, understanding all cost components and potential hidden expenses prevents budget surprises that could derail critical projects.
At ResolveMass Laboratories Inc., we’ve validated hundreds of GC-MS methods across diverse applications and regulatory requirements. Our transparent pricing, efficient processes, and deep regulatory expertise ensure you receive maximum value from your validation investment. We understand that every project has unique requirements and budget constraints, and we work collaboratively to deliver compliant, high-quality validations that support your business objectives.
The true cost of validation extends beyond immediate laboratory expenses to include the value of regulatory approval, product quality assurance, and competitive advantage. By investing in rigorous method validation with qualified partners, you protect patient safety, ensure product quality, and position your organization for long-term success in increasingly competitive markets.
FAQs on GC-MS Method Validation Cost:
Contact ResolveMass Laboratories Inc. with these key details for a precise quote:
-Analyte information: Number of compounds, chemical properties
-Matrix description: Sample type and complexity
-Validation scope: Parameters required by your regulatory agency
-Timeline requirements: Standard or expedited turnaround
-Documentation level: Internal use or regulatory submission
-Special requirements: Client-specific formatting, multi-site needs
Standard payment terms for GC-MS method validation:
-30-50% deposit upon protocol approval
-25-30% at validation completion (before final report)
-Remaining 20-25% upon report delivery
-Net 30 terms for established clients
-Volume discounts for multi-project commitments
Yes, through strategic approaches that maintain compliance:
-Risk-based validation focusing on critical parameters
-Phased validation spreading investment over time
-Platform approaches for similar methods
-Client-supplied materials where appropriate
-Leveraging developmental data scientifically
-Efficient project scoping preventing unnecessary testing
Reference
- Critical Aspects of Product Development: Analytical Method Development and Validation.https://newedgeoverseas.com/blog/pharmaceutical-companies-of-product-development-analytical-method-development-and-validation/
- Importance of analytical method validation in pharmaceutical research.https://www.wisdomlib.org/science/journal/world-journal-of-pharmaceutical-research/d/doc1382124.html
- A Review on Analytical Method Development andValidation.https://ijprajournal.com/issue_dcp/A%20Review%20on%20Analytical%20Method%20Development%20andValidation%20(With%20Case%20Study).pdf

