Introduction to Nitrosamine Testing in Desipramine
Nitrosamine Testing in Desipramine plays a crucial role in ensuring the safety, compliance, and long-term market approval of this tricyclic antidepressant. Global health regulators, including the U.S. FDA, EMA, and Health Canada, have strengthened their guidelines for nitrosamine impurities, making testing and risk control a mandatory part of the manufacturing process. Both the active pharmaceutical ingredient (API) stage and the finished product stage must be carefully evaluated to detect possible nitrosamine contamination and nitrosamine drug-substance-related impurities (NDSRIs).
At ResolveMass Laboratories Inc., we use advanced testing methods and regulatory expertise to detect and measure even trace levels of nitrosamines in Desipramine. This ensures accurate reports for submission to authorities, supports patient safety, and safeguards brand reputation in today’s quality-focused market.
Understanding the Synthesis of Desipramine
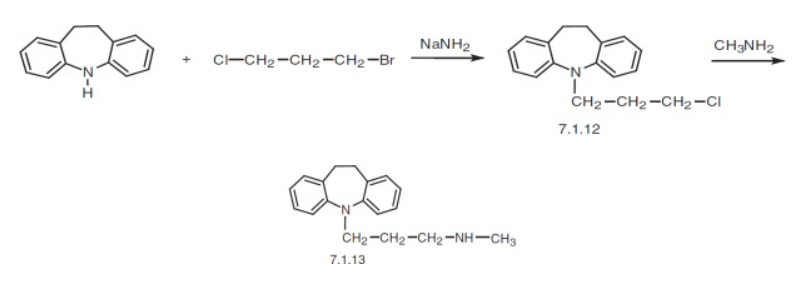
Desipramine (C₁₈H₂₂N₂) is obtained by first forming the tertiary amine imipramine through N‑alkylation of iminodibenzyl (5H‑dibenz[b,f]azepine) with 3‑dimethylaminopropyl chloride. The tertiary center is then selectively N‑demethylated to furnish desipramine as the secondary amine within the rigid tricyclic framework.
From a nitrosamine‑risk perspective, this route concentrates attention on two points: (1) the presence of secondary amine functionality in the final API, which is intrinsically nitrosation‑prone in the presence of nitrosating species (e.g., nitrite‑derived), and (2) the dimethylamino side‑chain chemistry introduced during the iminodibenzyl alkylation and consumed during N‑demethylation. Adventitious nitrite contacting secondary amines (desipramine) or dialkylamine residues related to the 3‑dimethylaminopropyl fragment can yield N‑nitroso species (notably NDMA from dimethylamine). Accordingly, tight control of nitrite ingress and amine carryover specifically around the iminodibenzyl alkylation and the subsequent N‑demethylation steps is critical to minimizing nitrosamine formation.
Elsevier. (n.d.). Desipramine. In ScienceDirect Topics: Chemistry. Retrieved August 15, 2025, from https://www.sciencedirect.com/topics/chemistry/desipramine
Nitrosamine Risk Assessment in Desipramine API Synthesis
In the described manufacturing route, desipramine is obtained by N-alkylation of iminodibenzyl with 3-dimethylaminopropyl chloride to produce imipramine, followed by selective N-demethylation. Two points in this sequence present specific nitrosamine risks:
- N-Nitrosodesipramine (NDesi): The final API contains a secondary amine that can undergo nitrosation if exposed to nitrite contaminants, particularly in acidic environments.
- N-Nitrosodimethylamine (NDMA): During the initial alkylation step, degradation of the 3-dimethylaminopropyl side chain or its by-products can release dimethylamine, which may be nitrosated to NDMA in the presence of nitrosating agents.
Given these specific risks, raw material nitrite levels, side-product amine content, and potential nitrosating species must be tightly controlled during both the alkylation and N-demethylation stages to minimize nitrosamine formation.
Reference: Nitrosamine Impurities in Pharmaceuticals
Nitrosamine Risk Assessment in Desipramine Finished Products
Following API production via N-alkylation of iminodibenzyl with 3-dimethylaminopropyl chloride and subsequent N-demethylation, two nitrosamine species remain relevant during formulation and packaging:
- N-Nitrosodesipramine (NDesi): The secondary amine in desipramine can undergo nitrosation if residual nitrites are present in excipients, particularly under elevated temperature or humidity during tablet compression or coating.
- N-Nitrosodimethylamine (NDMA): Trace dimethylamine remaining from the API synthesis can react with nitrites introduced via excipients to form NDMA.
To limit formation of these compounds in finished products, excipient nitrite levels should be tightly controlled, processing conditions kept mild where possible, and packaged products stored under low-moisture, temperature-stable conditions. Routine nitrosamine testing of final batches provides confirmation that these risks remain within acceptable limits.
Reference: Nitrosamine Risk Assessment Guide
Known Nitrosamine Impurities in Desipramine
For desipramine manufactured via N-alkylation of iminodibenzyl with 3-dimethylaminopropyl chloride followed by N-demethylation, the nitrosamines of primary concern are:
- N-Nitrosodesipramine (CAS 7521-26-0) – formed by nitrosation of the secondary amine in the API.
- N-Nitrosodimethylamine (NDMA) – potentially generated from dimethylamine residues originating from the 3-dimethylaminopropyl side chain or produced during N-demethylation.
Both must be quantified against the acceptable intake (AI) limits established by international regulatory agencies, which are typically in the nanogram-per-day range.
Each of these impurities must be evaluated against acceptable intake (AI) limits as per Health Canada nitrosamine limits.
Conclusion: Ensuring Safety Through Nitrosamine Testing in Desipramine
In the manufacturing route involving N-alkylation of iminodibenzyl with 3-dimethylaminopropyl chloride and subsequent N-demethylation, the nitrosamine impurities requiring the greatest attention are N-Nitrosodesipramine, arising from the secondary amine in the API, and N-Nitrosodimethylamine (NDMA), which can form from dimethylamine residues generated during side-chain introduction or N-demethylation.
By focusing testing and controls on these specific nitrosamines, monitoring raw materials for nitrite contamination, and managing processing and storage conditions to prevent secondary amine nitrosation, manufacturers can ensure desipramine batches remain within the nanogram-level acceptable intake limits defined by regulatory authorities.
ResolveMass Laboratories Inc. provides cutting-edge nitrosamine testing services in Canada, ensuring your products meet regulatory standards while delivering safe, high-quality medicines to patients.
Contact ResolveMass today-
FAQs – Nitrosamine Testing in Desipramine
The biggest concern is N-Nitrosodesipramine, which forms when the secondary amine in Desipramine reacts with nitrosating agents such as nitrite. This reaction can occur at various stages of manufacturing if proper controls are not in place, making targeted monitoring essential.
Absolutely. If recycled solvents contain trace amines or nitrites, they can become direct precursors for nitrosamine formation. Without strict purification and testing, these contaminants can compromise product safety and compliance.
Excipients such as starch, microcrystalline cellulose (MCC), and certain lubricants can contain residual nitrites. When combined with Desipramine’s reactive amine, these nitrites may trigger nitrosamine formation, especially during heat or moisture exposure.
Yes. Even after manufacturing, factors like oxidation, humidity, and elevated temperature can cause nitrosamine levels to increase over time. Proper packaging and controlled storage conditions are critical to prevent this.
High-sensitivity techniques like LC-MS/MS and GC-MS are widely used to detect and quantify N-Nitrosodesipramine. These methods can identify extremely low impurity levels, meeting strict global regulatory limits.
Once nitrosamines are formed, removal is technically possible but highly complex, time-consuming, and costly. Prevention through process design and contamination control is the most effective and economical approach.
Get in touch with us
References
- Elsevier. (n.d.). Desipramine. In ScienceDirect Topics: Chemistry. Retrieved August 15, 2025, from https://www.sciencedirect.com/topics/chemistry/desipramine
- U.S. Food and Drug Administration. (2024, September). Control of nitrosamine impurities in human drugs (Final revised guidance). U.S. Department of Health & Human Services. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/control-nitrosamine-impurities-human-drugs
- Health Canada. (2025, August 1). Nitrosamine impurities in medications: Guidance. Government of Canada. https://www.canada.ca/en/health-canada/services/drugs-health-products/compliance-enforcement/information-health-product/drugs/nitrosamine-impurities/medications-guidance.html


